Articles
- Page Path
- HOME > J Trauma Inj > Volume 37(1); 2024 > Article
-
Original Article
Usefulness of presepsin as a prognostic indicator for patients with trauma in the emergency department in Korea: a retrospective study -
Si Woo Kim, MD
 , Jung-Youn Kim, MD
, Jung-Youn Kim, MD , Young-Hoon Yoon, MD
, Young-Hoon Yoon, MD , Sung Joon Park, MD
, Sung Joon Park, MD , Bo Sun Shim, MD
, Bo Sun Shim, MD
-
Journal of Trauma and Injury 2024;37(1):13-19.
DOI: https://doi.org/10.20408/jti.2023.0061
Published online: January 12, 2024
- 676 Views
- 17 Download
Department of Emergency Medicine, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea
- Correspondence to: Jung-Youn Kim, MD Department of Emergency Medicine, Korea University Guro Hospital, Korea University College of Medicine, 148 Gurodong-ro, Guro-gu, Seoul 08308, Korea Tel: +82-2-2626-1561 Email: yellowwizard@hanmail.net
© 2024 The Korean Society of Traumatology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Purpose
- Trauma is an important public health concern, and it is important to increase the survival rate of patients with trauma and enable them to return to society in a better condition. Initial treatment in the emergency department (ED) is closely associated with the prognosis of patients with trauma. However, studies regarding laboratory biomarker tests that can help predict the prognosis of trauma patients are limited. Presepsin is a novel biomarker of inflammation that can predict a poor prognosis in patients with sepsis. This study aimed to determine whether presepsin could be used as a prognostic indicator in patients with polytrauma.
-
Methods
- The study included patients with trauma who had visited a single regional ED from November 2021 to January 2023. Patients who had laboratory tests in the ED were included and analyzed retrospectively through chart review. Age, sex, injury mechanism, vital signs, surgery, the outcome of ED treatment (admission, discharge, transfer, or death), and trauma scores were analyzed.
-
Results
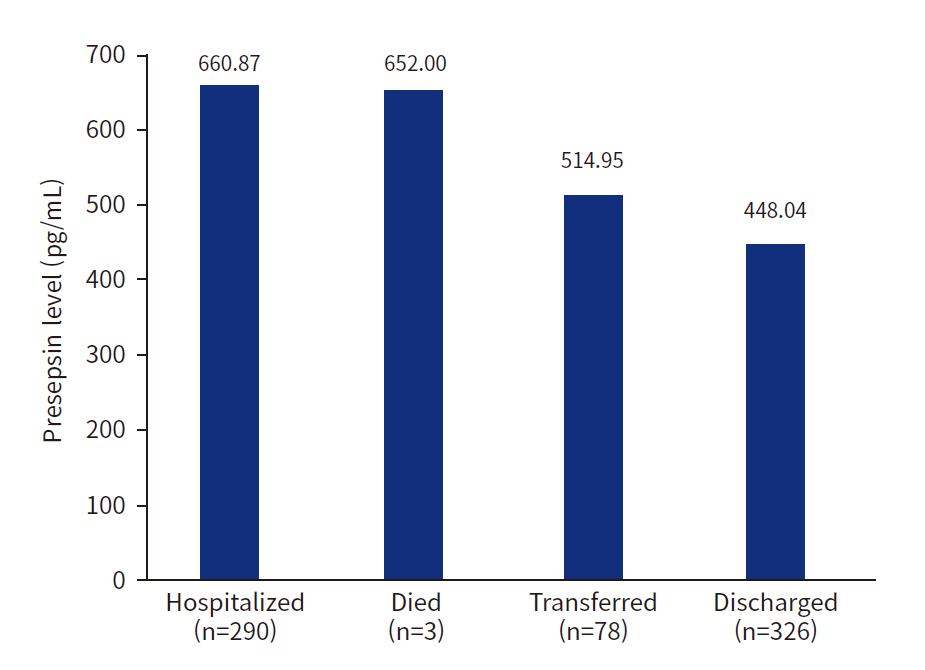
- Overall, 550 trauma patients were enrolled; 59.1% were men, and the median age was 64 years (interquartile range, 48.8–79.0 years). Patients in a hypotensive state (systolic blood pressure, <90 mmHg; n=39) had higher presepsin levels (1,061.5±2,522.7 pg/mL) than those in a nonhypotensive state (n=511, 545.7±688.4 pg/mL, P<0.001). Patients hospitalized after ED treatment had the highest presepsin levels (660.9 pg/mL), followed by those who died (652.0 pg/mL), were transferred to other hospitals (514.9 pg/mL), and returned home (448.0 pg/mL, P=0.041).
-
Conclusions
- Serum presepsin levels were significantly higher in trauma patients in a hypotensive state than in those in a nonhypotensive state. Additionally, serum presepsin levels were the highest in hospitalized patients with trauma, followed by those who died, were transferred to other hospitals, and returned home.
- Background
- Trauma is a significant public health issue, with high prevalence and mortality rates, especially among young individuals who are socially and economically active [1]. The effects of traumatic injuries are staggering, accounting for over 40 million emergency department (ED) visits each year in the United States alone [2]. On a global scale, these injuries result in an alarming annual death toll of approximately six million people. Trauma continues to be the leading cause of death for both children and adults under the age of 46 years, representing nearly half of all fatalities within these age groups [1,3,4]. Therefore, it is crucial to improve the survival rates of trauma patients and facilitate their return to society in an improved condition.
- Traumatic deaths are traditionally characterized by a trimodal pattern, with the initial treatment in the ED playing a significant role in the prognosis of trauma patients [5]. A variety of inflammatory cytokines contribute to traumatic deaths through the immune-inflammation cascade [4,6,7]. Despite the importance of this field, research is still limited, particularly in relation to laboratory biomarker tests that can predict the prognosis of trauma patients.
- Presepsin (soluble CD14 [sCD14] subtypes, discovered in 2004) is a novel biomarker of inflammation that can predict a poor prognosis in patients with sepsis [8–16]. As a receptor of the lipopolysaccharide-binding protein (LPS-LBP) complex, sCD14 can trigger a series of signal transduction pathways and inflammatory cascades, leading to a systemic inflammatory response [4,17–19]. Several clinical studies examining the relationship between sCD14 and sepsis have demonstrated that sCD14 levels significantly increase in patients with sepsis and septic shock, compared to healthy individuals [20,21]. However, the specificity of sCD14 is low, and its levels are also significantly elevated in patients with coronary heart disease, heart failure, and liver cirrhosis [11].
- Objectives
- This study aimed to determine whether presepsin could be used as a prognostic indicator in patients with polytrauma.
INTRODUCTION
- Ethics statement
- This study was approved by the Institutional Research Board of Korea University Guro Hospital (No. 2023GR0364). The requirement for informed consent from the participants was waived due to the retrospective nature of the study. The study adhered to the principles of the Declaration of Helsinki.
- Study design and setting
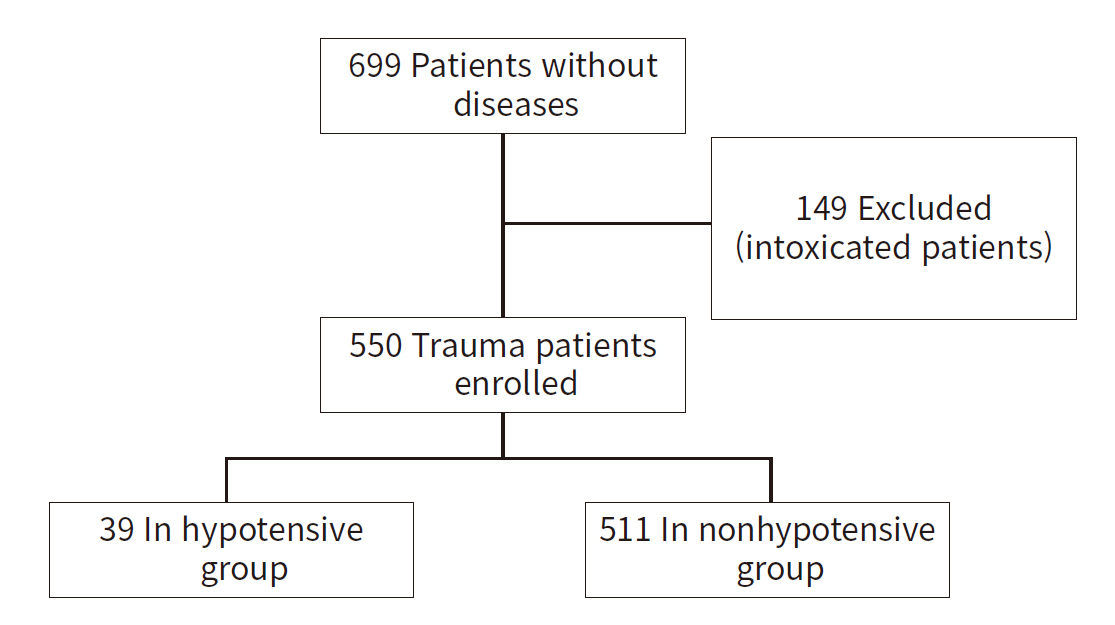
- This study was carried out at Korea University Guro Hospital (Seoul, Korea) which was recognized as a level I trauma center in 2016 and currently functions as a regional trauma center in the city. The research encompassed 699 patients without any preexisting diseases who visited the ED between November 2021 and January 2023. Of these, 149 patients who were intoxicated were excluded, leaving data from 550 trauma patients for analysis. Patients deemed to be under the influence of substances during ED triage were not included in the study. Most of these patients were intoxicated due to alcohol, drugs, pesticides, and other substances. The study focused on patients who required blood tests in the ED, thus excluding those who visited for minor injuries or simple treatments. Trauma was classicfied as a physical injury caused by traffic accidents, falls, blunt injury, penetrating injury, or other causes (Table 1).
- Clinical and laboratory variables were collected retrospectively from electronic medical and emergency medical services transport records. The baseline characteristics were collected at the time of ED triage and included age, sex, injury mechanism, vital signs, Glasgow Coma Scale score, surgery, and ED treatment results. All laboratory data, including presepsin, were collected at the ED at the initial presentation, and the data included the complete blood count and levels of lactic acid and inflammatory markers, including C-reactive protein (CRP), procalcitonin (PCT), and presepsin. We also calculated the Revised Trauma Score (RTS), Injury Severity Score (ISS), and Trauma Injury Severity Score (TRISS) as indicators of severity. Details about the outcomes of ED treatment (admission, discharge, transfer, and death) were also collected. Patients were divided into a hypotension group (systolic blood pressure [SBP], <90 mmHg) and a nonhypotension group (SBP, >90 mmHg) based on the SBP measured from the initial vital signs taken when the patient first presented to the ED.
- Statistical analysis
- IBM SPSS ver. 20.0 (IBM Corp) was used for the statistical analyses. The continuous variables are expressed as medians (interquartile ranges [IQRs]) or means±standard deviations. Categorical variables are expressed as frequencies (percentages). In this study, analysis of variance and the t-test were used for data analysis. The t-test was used to compare mean differences between the two groups. Statistical significance was set at P<0.05.
METHODS
- This study included 699 patients without diseases who visited the ED during the study period. A total of 149 intoxicated patients were excluded, and data from 550 patients with trauma were analyzed (Fig. 1). Overall, 550 trauma patients were enrolled, of whom 59.1% were men, and the median age was 64 years (IQR, 48.8–79.0 years). Thirty-nine patients were in a hypotensive state and 511 were not. The proportion of men among patients with and without a hypotensive state was 64.1% and 58.7%, respectively. The median age of patients with and without hypotensive state was 59 years (IQR, 39–73 years) and 65 years (IQR, 50–79 years), respectively. No significant difference between the two groups in terms of initial SBP (P=0.166), initial body temperature (P=0.114), and PCT levels (P=0.749) was observed. However, the initial respiratory rate (P<0.001) and heart rate (P=0.049) differed significantly between the two groups. In addition, the ISS (P<0.001), RTS (P<0.001), and TRISS (P<0.001) showed significant between-group differences (Table 1).
- Patients in a hypotensive state (SBP, <90 mmHg; n=39) had higher presepsin levels (mean, 1,061.41 pg/mL; median, 566 pg/mL; IQR, 413–789 pg/mL) than those who were not (n=511; mean, 545.7 pg/mL; median, 404 pg/mL; IQR, 293–544 pg/mL; P<0.001) (Fig. 2, Table 1).
- Patients hospitalized after ED treatment had the highest presepsin levels (660.87 pg/mL), followed by those who died (652.00 pg/mL), were transferred to other hospitals (514.95 pg/mL), and returned home (448.04 pg/mL, P=0.041) (Fig. 3).
- Fig. 4 shows the receiver operating characteristic (ROC) curve between presepsin level and patients with and without a hypotensive state, and the area under the ROC curve was 0.669.
- Although not statistically significant, patients who underwent surgery had higher presepsin levels (630.2 pg/mL) than those who did not (523.8 pg/mL, P=0.526). No significant difference was observed between patients with ISS above 15 (569.1 pg/mL) or below 15 (513.6 pg/mL, P=0.887).
RESULTS
- To the best of our knowledge, this study is the first to validate the usefulness of presepsin levels in trauma patients, marking its significance. Our findings revealed significant differences in presepsin levels depending on whether trauma patients were in a hypotensive state or not. Presepsin, a marker for the severity of various medical conditions, is typically considered to fall within a normal range of approximately 500 ng/L. In line with previous studies, our trauma patients in a hypotensive state displayed presepsin levels nearly double those of patients not in a hypotensive state. Importantly, presepsin levels were significantly higher in patients with severe outcomes, such as hospitalization or death, compared to those with less severe outcomes, like hospital discharge or transfer. Most patients transferred to other hospitals were transferred due to the perceived minor severity of their injuries. This marked difference suggests the potential of presepsin as a prognostic marker in trauma patients. Given these insights, presepsin shows promise as a valuable prognostic tool for traumatic injury.
- As noted earlier, presepsin is a novel biomarker of inflammation that has been widely studied recently and has high sensitivity and specificity for the diagnosis of bacterial infection [21–24]. This study demonstrated that elevated presepsin levels not only indicate a high suspicion of sepsis, but also predict a poor prognosis for patients with trauma. We also found that along with presepsin levels (P<0.001), the initial lactic acid levels (P<0.001) showed significant differences between patients with and without a hypotensive state. However, no significant differences between the two groups with respect to the initial PCT levels (P=0.749) and CRP levels (P=0.425) were observed. Further research is required to validate the potential use of CRP, PCT, and lactic acid levels as prognostic indicators in trauma patients. When assessing the relationship between inflammatory markers and prognosis, changes observed during follow-up can be as important as the initial increase. However, this study focused on the correlation between initial factors and prognosis in an ED setting, and therefore did not include data from several days post–initial assessment.
- Patients with polytrauma can die through various pathophysiological pathways, which may or may not involve infectious components [25]. In an effort to maintain homeostasis, a trauma patient's body triggers an inflammatory cascade, which could potentially provide predictive indicators of outcomes through associated biomarkers. This same inflammatory cascade is activated in order to maintain physiological equilibrium during sepsis, infection, and trauma [4]. Given the current research on presepsin as an inflammatory biomarker in patients with sepsis and infection, we hypothesized that its levels might also be elevated in trauma patients, which led us to conduct this study.
- According to a study by Zhang et al. [16], presepsin was effective for the diagnosis of sepsis, but has limitations as a standalone rule-out marker. In our study, presepsin exhibited differences between trauma patients with and without a hypotensive state and correlated with ED outcomes. However, further investigations, including comparisons with other markers and multiple regression analyses, are required.
- Kang et al. [4] categorized trauma patients into two groups: those with infections and those without. They found that plasma presepsin levels within the first 3 days of admission were significantly higher only in the group with infections. Although presepsin shows promise as a superior biomarker for early differentiation of infection in trauma patients, the increase in PCT and CRP levels, as well as white blood cell counts due to trauma stress, necessitates caution when using these indicators for infection diagnosis. As Kang et al. [4] reported, markers that are elevated in infection and sepsis could also rise due to noninfectious trauma stress, thus requiring differentiation. Our study measured presepsin levels in all trauma patients, regardless of their infection status, and confirmed its elevation even in cases of simple trauma. Kang et al. [4] proposed that presepsin might react specifically in patients with trauma-related infections. However, our study suggests that presepsin might also respond to traumatic stress that is not related to infection. The commonly used biomarker CRP typically indicates an inflammatory response, but it is not specific to either infection or trauma. Conversely, PCT is often used as a biomarker for detecting infection, particularly bacterial infection, rather than trauma itself. In this study, we sought to determine whether presepsin could serve as a biomarker for trauma-induced hypotension, irrespective of infection.
- In humans, presepsin is primarily produced by monocytes and macrophages [4,26]. This biomarker, along with others and white blood cells, displays unique elevation characteristics due to their individual production mechanisms. While white blood cells are the primary effector cells in the inflammatory response following trauma, their increase can also be a reaction to the stress induced by trauma, rather than a specific indication of a post-traumatic infection [4,27]. Levels of CRP and PCT act as indirect markers of the host-pathogen response, as they can rise in response to lipopolysaccharide or specific cytokines [4,28]. Procalcitonin is mainly released by the neuroendocrine cells of various organs, while CRP is predominantly produced by hepatocytes [4,29]. Consequently, both PCT and CRP levels can escalate during the acute phase of systemic inflammation, which includes infection and trauma.
- Limitations
- This study had some limitations. First, it was a retrospective analysis carried out at a single tertiary trauma center, which may limit the generalizability of the results. Second, the study period was approximately 1 year and 2 months, potentially restricting the size of the patient cohort. Third, there was a lack of clinical data related to the course of treatment for trauma patients, including factors such as the length of hospital stay. Fourth, the study only included the initial lab results for presepsin levels, and thus, we were unable to confirm any pattern of fluctuation in relation to the trimodal phases of trauma. To address these limitations and strengthen our findings, further research involving larger multicenter prospective studies and the integration of comprehensive clinical data is recommended.
- Conclusions
- Serum presepsin levels were significantly higher in patients with trauma in a hypotensive state than in those in a nonhypotensive state. In addition, serum presepsin levels were the highest in hospitalized trauma patients, followed by those who died, were transferred to other hospitals, and returned home.
DISCUSSION
-
Author contributions
Conceptualization: all authors; Data curation: SWK; Formal analysis: JYK; Methodology: SWK; Project administration: all authors; Visualization: SWK; Writing–original draft: SWK; Writing–review & editing: all authors. All authors read and approved the final manuscript.
-
Conflicts of interest
The authors have no conflicts of interest to declare.
-
Funding
The authors received no financial support for this study.
-
Data availability
Data analyzed in this study are available from the corresponding author upon reasonable request.
ARTICLE INFORMATION

- 1. Prin M, Li G. Complications and in-hospital mortality in trauma patients treated in intensive care units in the United States, 2013. Inj Epidemiol 2016;3:18. ArticlePubMedPMCPDF
- 2. Tintinalli JE, Ma O, Yealy DM, et al. Tintinalli’s emergency medicine: a comprehensive study guide. 2nd ed. McGraw Hill; 2020.
- 3. Hokkam E, Gonna A, Zakaria O, El-Shemally A. Trauma patterns in patients attending the Emergency Department of Jazan General Hospital, Saudi Arabia. World J Emerg Med 2015;6:48–53. ArticlePubMedPMC
- 4. Kang J, Gong P, Zhang XD, Wang WJ, Li CS. Early differential value of plasma presepsin on infection of trauma patients. Shock 2019;52:362–9. ArticlePubMed
- 5. Demetriades D, Kimbrell B, Salim A, et al. Trauma deaths in a mature urban trauma system: is "trimodal" distribution a valid concept. J Am Coll Surg 2005;201:343–8. ArticlePubMed
- 6. Marshall JC, Reinhart K, International Sepsis Forum. Biomarkers of sepsis. Crit Care Med 2009;37:2290–8. ArticlePubMed
- 7. Chaudhry H, Zhou J, Zhong Y, et al. Role of cytokines as a double-edged sword in sepsis. In Vivo 2013;27:669–84. PubMed
- 8. Zanoni I, Granucci F. Role of CD14 in host protection against infections and in metabolism regulation. Front Cell Infect Microbiol 2013;3:32. ArticlePubMedPMC
- 9. Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 1990;249:1431–3. ArticlePubMed
- 10. Schumann RR. Function of lipopolysaccharide (LPS)-binding protein (LBP) and CD14, the receptor for LPS/LBP complexes: a short review. Res Immunol 1992;143:11–5. ArticlePubMed
- 11. Zou Q, Wen W, Zhang XC. Presepsin as a novel sepsis biomarker. World J Emerg Med 2014;5:16–9. ArticlePubMedPMC
- 12. Meuleman P, Steyaert S, Libbrecht L, et al. Human hepatocytes secrete soluble CD14, a process not directly influenced by HBV and HCV infection. Clin Chim Acta 2006;366:156–62. ArticlePubMed
- 13. Su GL, Dorko K, Strom SC, Nussler AK, Wang SC. CD14 expression and production by human hepatocytes. J Hepatol 1999;31:435–42. ArticlePubMed
- 14. Mussap M, Noto A, Fravega M, Fanos V. Soluble CD14 subtype presepsin (sCD14-ST) and lipopolysaccharide binding protein (LBP) in neonatal sepsis: new clinical and analytical perspectives for two old biomarkers. J Matern Fetal Neonatal Med 2011;24 Suppl 2:12–4. ArticlePubMed
- 15. Yaegashi Y, Shirakawa K, Sato N, et al. Evaluation of a newly identified soluble CD14 subtype as a marker for sepsis. J Infect Chemother 2005;11:234–8. ArticlePubMed
- 16. Zhang J, Hu ZD, Song J, Shao J. Diagnostic value of presepsin for sepsis: a systematic review and meta-analysis. Medicine (Baltimore) 2015;94:e2158PubMedPMC
- 17. Chenevier-Gobeaux C, Borderie D, Weiss N, Mallet-Coste T, Claessens YE. Presepsin (sCD14-ST), an innate immune response marker in sepsis. Clin Chim Acta 2015;450:97–103. ArticlePubMed
- 18. Ackland GL, Prowle JR. Presepsin: solving a soluble (CD14) problem in sepsis? Intensive Care Med 2015;41:351–3. ArticlePubMedPDF
- 19. Jersmann HP. Time to abandon dogma: CD14 is expressed by non-myeloid lineage cells. Immunol Cell Biol 2005;83:462–7. ArticlePubMedPDF
- 20. Endo S, Suzuki Y, Takahashi G, et al. Usefulness of presepsin in the diagnosis of sepsis in a multicenter prospective study. J Infect Chemother 2012;18:891–7. ArticlePubMed
- 21. Carpio R, Zapata J, Spanuth E, Hess G. Utility of presepsin (sCD14-ST) as a diagnostic and prognostic marker of sepsis in the emergency department. Clin Chim Acta 2015;450:169–75. ArticlePubMed
- 22. Ulla M, Pizzolato E, Lucchiari M, et al. Diagnostic and prognostic value of presepsin in the management of sepsis in the emergency department: a multicenter prospective study. Crit Care 2013;17:R168. ArticlePubMedPMC
- 23. Liu B, Chen YX, Yin Q, Zhao YZ, Li CS. Diagnostic value and prognostic evaluation of presepsin for sepsis in an emergency department. Crit Care 2013;17:R244. ArticlePubMedPMC
- 24. Romualdo LG, Torrella PE, Gonzalez MV, et al. Diagnostic accuracy of presepsin (soluble CD14 subtype) for prediction of bacteremia in patients with systemic inflammatory response syndrome in the Emergency Department. Clin Biochem 2014;47:505–8. ArticlePubMed
- 25. Desborough JP. The stress response to trauma and surgery. Br J Anaesth 2000;85:109–17. ArticlePubMed
- 26. Arai Y, Mizugishi K, Nonomura K, Naitoh K, Takaori-Kondo A, Yamashita K. Phagocytosis by human monocytes is required for the secretion of presepsin. J Infect Chemother 2015;21:564–9. ArticlePubMed
- 27. Egger G, Aigner R, Glasner A, Hofer HP, Mitterhammer H, Zelzer S. Blood polymorphonuclear leukocyte migration as a predictive marker for infections in severe trauma: comparison with various inflammation parameters. Intensive Care Med 2004;30:331–4. ArticlePubMedPDF
- 28. Salluh JIF, Souza-Dantas VC, Povoa P. The current status of biomarkers for the diagnosis of nosocomial pneumonias. Curr Opin Crit Care 2017;23:391–7. ArticlePubMed
- 29. Matwiyoff GN, Prahl JD, Miller RJ, et al. Immune regulation of procalcitonin: a biomarker and mediator of infection. Inflamm Res 2012;61:401–9. ArticlePubMedPDF
REFERENCES
Figure & Data
References
Citations

- Figure
- Related articles
-
- Angioembolization performed by trauma surgeons for trauma patients: is it feasible in Korea? A retrospective study
- Comparison of pediatric injury patterns before and during the COVID-19 pandemic in Korea: a retrospective study
- A decade of treating traumatic sternal fractures in a single-center experience in Korea: a retrospective cohort study
- Clinical characteristics and mortality risk factors among trauma patients by age groups at a single center in Korea over 7 years: a retrospective study
- Epidemiology and outcomes of patients with penetrating trauma in Incheon Metropolitan City, Korea based on National Emergency Department Information System data: a retrsopective cohort study
 KST
KST



 PubReader
PubReader ePub Link
ePub Link Cite
Cite