The management of Pancreatic fistula Complicated by Gastric fistulation following Emergency Splenectomy
Article information
Abstract
Pancreatic and gastric fistulas are rare complications of emergency splenectomy, and it is extremely rare for a pancreatic fistula to be further complicated by a fistulation into the stomach. Here, we present a case of pancreatogastric fistula in a 60-year-old man who experienced polytrauma due to a blunt mechanism. He underwent emergency splenectomy for splenic injury and developed a pancreatic fistula as a complication. A percutaneous endoscopic procedure was performed to drain the fistula, after which he developed a pancreatogastric fistula as a further complication. A double-pigtail stent was inserted via gastroscopy into the fistula tract to allow internal drainage of the pancreatic collection into the stomach cavity. When a pancreatic fistula is complicated by gastric fistulation, endoscopic stenting of the pancreatogastric fistula tract for internal drainage is an effective treatment option.
INTRODUCTION
Pancreatic fistula formation is a rare complication following splenectomy in patients with traumatic injuries [1]. Management and treatment approaches range from supportive care, endoscopic therapy, and percutaneous drainage to surgical therapy [1,2]. Gastric fistula following splenectomy is uncommon, but can be caused by gastric wall necrosis due to suture ligation of the short gastric artery to the gastric tissue [3]. It is extremely rare for these two complications to co-occur as a pancreatogastric fistula. To our knowledge, no such cases have been reported in patients after emergency splenectomy, although this phenomenon has been described in patients with chronic pancreatitis and intraductal papillary mucinous neoplasm [4]. Herein, we describe the case of a pancreatogastric fistula in a 60-year-old man after emergency splenectomy for a splenic injury due to blunt trauma. He developed a pancreatic fistula as a complication, and later, the endoscopic drainage procedure for the pancreatic fistula resulted in a pancreatogastric fistula. We discuss the available evidence on the management of post-splenectomy pancreatic fistula and the treatment of pancreatogastric fistula, which is an unusual complication.
CASE REPORT
A 60-year-old Chinese man involved in a motor vehicle accident presented with reduced consciousness, shortness of breath, and abdominal pain. His blood pressure on arrival was 166/87, his heart rate was 101 bpm, and his oxygen saturation was 94% under high-flow oxygen. His Glasgow Coma Scale score was 10/15, with unequal reactive pupils. Upon palpation, the abdomen was soft, but mildly distended. He was intubated due to reduced consciousness and poor oxygenation. Arterial blood gas analysis revealed a PaO2 of 282 mmHg, a pH of 7.366, a pCO2 of 36 mmHg, a bicarbonate level of 21.1 mmol/L, and a base deficit of -4.3 mmol/L. The patient’s haemoglobin level was 14.3 g/dL and platelet count was 147×109/L. Chest radiography revealed fractures of multiple left ribs and the clavicle. An abdominal Focused Assessment with Sonography for Trauma scan showed free fluid over the perihepatic and perisplenic spaces. Computed tomography (CT) of the thorax, abdomen, and pelvis revealed grade 2 splenic injury and fracture of the left third through 10th ribs with lung contusions. Brain CT revealed right temporal acute subdural haemorrhage of 0.5 cm thickness with adjacent contusions, the largest of which was approximately 2×1 cm. There was associated right frontotemporoparietal subarachnoid haemorrhage and left parietal subgaleal haematoma. The patient received intensive care with cerebral protection.
Despite optimal resuscitation with blood transfusions, there was a gradual decrease in the patient’s haemoglobin level to 8.8 g/dL. In light of the dropping haemoglobin level and the lack of an interventional radiology service for angioembolization and severe traumatic brain injury, an emergency exploratory laparotomy was performed. Intraoperatively, the spleen was identified to have a grade 3 splenic injury with multiple splenic lacerations measuring 1-3 cm at its anterior and superior borders. The spleen was mobilized from its lateral ligamentous attachment and brought medially to the wound. The hilar vessels were clamped and ligated with absorbable 2-0 sutures and stitch-tied with Prolene 2-0. A Surgicell (ETHICON, Neuchâtel, Switzerland) haemostatic device was placed around the hilar ligature. A soft drain was placed over the splenic bed and drained 100 to 300 mL per day. The amylase level in the drainage fluid amylase level was 41,180 U/L. The drain was removed on day 7 following splenectomy when it stopped flowing and the amylase level dropped to 17,506 U/L. The patient developed fever following drain removal. Repeat ultrasonography and abdominal CT revealed a left subphrenic collection (Fig. 1). The white cell count had increased to 29.2×109/L, with a C-reactive protein level of 242 U/L. Ultrasound-guided percutaneous drainage of the collection was performed with a 12-Fr pigtail catheter. Tracheostomy was performed to facilitate early weaning from the ventilator. Tazobactam and piperacillin were prescribed empirically, as the culture yielded mixed patterns of microorganisms.
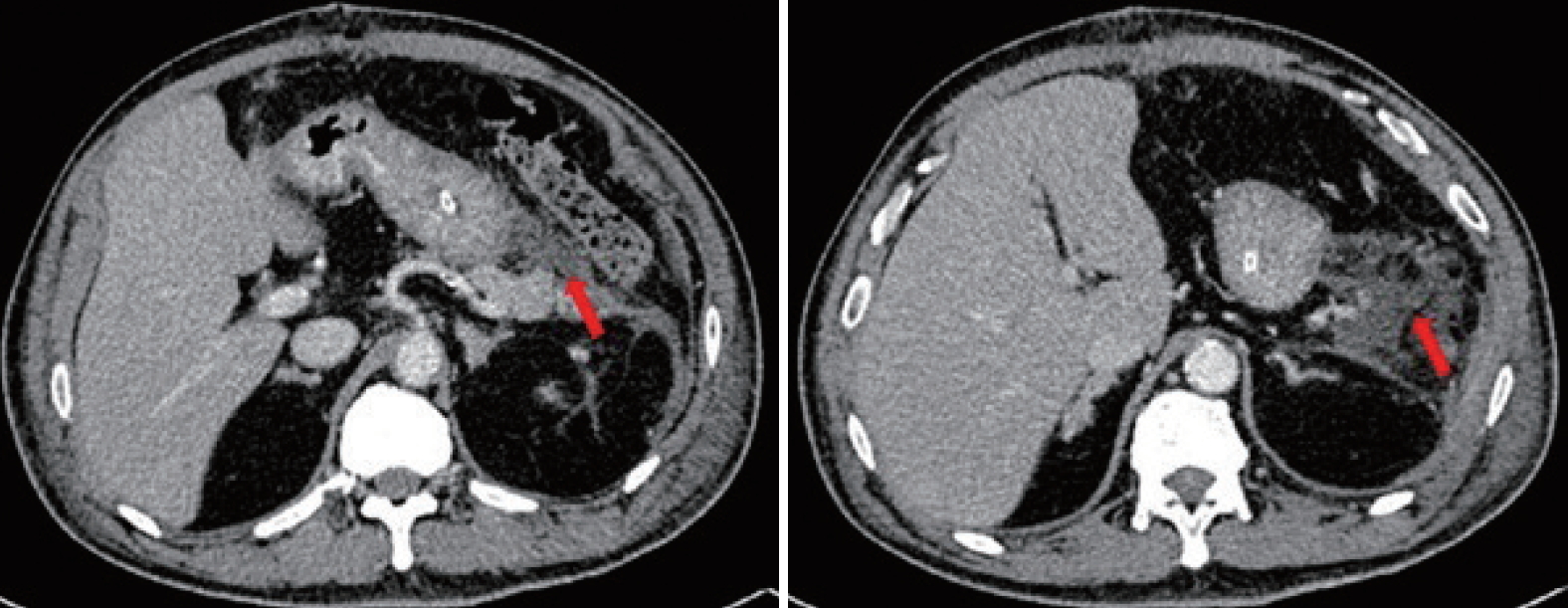
Computed tomography performed 9 days post-splenectomy showing a collection (arrows) at the splenic bed/pancreatic tail with a close relationship to the greater curvature of the stomach.
The percutaneous abdominal drain persistently drained 300 to 800 mL of yellowish fluid per day due to a pancreatic fistula. An endoscopic retrograde cholangiopancreatography with pancreatography was performed, and revealed contrast leakage at the pancreatic tail. A pancreatic stent was inserted during the procedure. The percutaneous pig-tail drain into the pancreatic fistula collection was upsized to 36-Fr due to poor drainage flow. Repeated abdominal CT after upsizing of the drain revealed residual collection with a close relationship of the rubber drain to the gastric fundus (Fig. 2). Two months post-splenectomy, endoscopic washout was performed with a therapeutic gastroscope via the drain track. A 36-Fr Portex (silicone-drain; Primed, Halberstadt, Germany) tube was inserted following the procedure. Three days after insertion of the Portex (Primed) tube, undigested food was present within the drainage tube.

The rubber drain (blue arrows) was abutting onto the gastric fundus (red arrows). The image on the left is cephalad to the right.
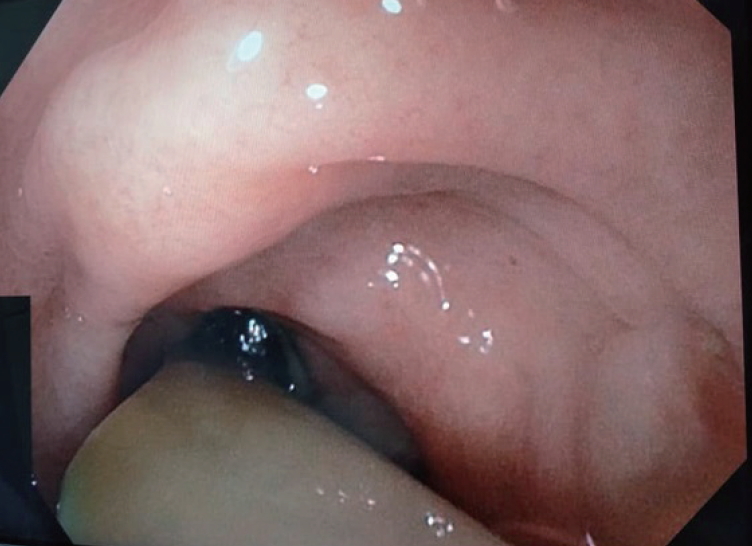
Gastroduodenoscopy was performed and revealed that the drainage tube penetrated into the gastric body (Fig. 3). The pancreatic stent was removed during the endoscopy, and a 10-Fr double-pigtail stent was placed at the pancreatogastric fistula opening. The rubber tube drain within the fistula was removed and the cutaneous drain opening was contained by a stoma device. Following this, the patient remained afebrile and with no peritonism for 3 days, was started on a solid diet, and was discharged home in good condition. At a 1-month follow-up, the patient remained well, with obliteration of the cutaneous opening.
DISCUSSION
The International Study Group for Pancreatic Fistula defined the diagnosis of a postoperative pancreatic fistula by the clinical criteria of the presence of any measurable volume of drainage after postoperative day 3 with an amylase level more than three times the upper limit of the normal serum value, along with clinical symptoms and signs of local infection or sepsis [1]. Pancreatic fistulas commonly form due to procedures such as pancreatic or gastric resection and percutaneous drainage of a pancreatic pseudocyst, pancreatic abscess, or organised necrosis. It is less common following splenectomy, and even rarer in a trauma setting [1,5]. In a study of patients with ovarian cancer who underwent cytoreductive surgery, pancreatic fistula was reported in up to 29% of patients who underwent splenectomy [3]. In contrast, very few cases of pancreatic fistula have been reported following emergency splenectomy [1,6,7]. This may be due to the current paradigm of nonoperative management for blunt splenic injuries, especially in developed nations with satisfactory intensive care resources.
Based on the previous literature, the risk factors for pancreatic fistula formation are body mass index (BMI), perirenal fat thickness, pancreatic duct width on CT and upon surgery, and gland firmness [8]. Other than the patient’s BMI, these risk factors are impractical in trauma settings, as trauma victims usually have a normal pancreas.
Several technical options have been described to prevent pancreatic fistula, including the use of a haemostatic sealing device (Hemopatch) [9], the use of fibrin glue/polyglycolic acid [10], prophylactic usage of somatostatin and its analogues [11], a falciform ligament pedicle flap [12], and prophylactic pancreatic stenting [13]. The options have most frequently been described for elective pancreatic resection. In emergency splenectomy with possible pancreatic tail injury either due to dissection or primary injury, the aforementioned options remain to be explored in a future study. Nonetheless, the authors would like to highlight the point that preventing pancreatic tail injury by adhering to operative rules of ligating hilar vessels close to the spleen would be better than treating a pancreatic fistula complication.
Four factors may predispose individuals to the formation of a gastric fistula following splenectomy: direct surgical trauma to the gastric wall, generalized arteriosclerotic disease, haematoma in the gastrosplenic omentum, and reflection of gastric muscle fibres into the gastrosplenic ligament [14]. A case of the drainage tube causing stress fistulation of a pancreatic fistula collection into the stomach has been reported in the English-language literature [3]. In our case, a pancreatogastric fistula may have formed due to a combination of a few factors, including the rubber drain placement, the patient’s elderly age, the possibility of arteriosclerotic vessels, and surrounding inflammation. The formation of a fistula between the stomach and the pancreas, resulting in a gastropancreatic or pancreatogastric fistula, has never before been reported in a post-splenectomy patient. Such fistulas have only been reported in patients with pancreatic lesions, such as an intraductal papillary mucinous neoplasm [4,15], in whom they may be treated with a conservative approach, endoscopic stenting, or surgically with duodenal pancreatectomy [4]. In our case, the pancreatogastric fistula was treated with endoscopic stenting. Gastroscopy was done and internal drainage was achieved between the gastric and pancreatic collection with a double-pigtail stent, after which the patient recovered uneventfully.
In conclusion, when a pancreatic fistula is complicated with gastric fistulation, endoscopic stenting of the pancreatogastric fistula tract for internal drainage is a safe and effective option. The external percutaneous drain can be removed following successful internal drainage.