Articles
- Page Path
- HOME > J Trauma Inj > Volume 35(3); 2022 > Article
-
Original Article
Epidemiology and clinical characteristics of posttraumatic hospitalized patients with symptoms related to venous thromboembolism: a single-center retrospective study -
Hyung Su Park, MD1
 , Sung Youl Hyun, MD2
, Sung Youl Hyun, MD2 , Woo Sung Choi, MD1
, Woo Sung Choi, MD1 , Jin-Seong Cho, MD3
, Jin-Seong Cho, MD3 , Jae Ho Jang, MD1
, Jae Ho Jang, MD1 , Jea Yeon Choi, MD3
, Jea Yeon Choi, MD3
-
Journal of Trauma and Injury 2022;35(3):159-167.
DOI: https://doi.org/10.20408/jti.2021.0052
Published online: June 10, 2022
- 1,945 Views
- 61 Download
1Department of Emergency Medicine, Gachon University Gil Medical Center, Incheon, Korea
2Department of Traumatology, Gachon University Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
3Department of Emergency Medicine, Gachon University Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
- Correspondence to Sung Youl Hyun, MD, Department of Traumatology, Gachon University Gil Medical Center, Gachon University College of Medicine, 21 Namdong-daero 774beon-gil, Namdong-gu, Incheon 21565, Korea Tel: +82-32-460-3010 E-mail: sungyoul@gilhospital.com
- Woo Sung Choi, MD, Department of Emergency Medicine, Gachon University Gil Medical Center, 21 Namdong-daero 774beon-gil, Namdong-gu, Incheon 21565, Korea Tel: +82-32-460-3901 E-mail: choiwoosung@gilhospital.com
Copyright © 2022 The Korean Society of Traumatology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Purpose
- The aim of this study was to investigate the epidemiology of trauma inpatients with venous thromboembolism (VTE) symptoms diagnosed using computed tomographic angiography (CTA) in Korea.
-
Methods
- In total, 7,634 patients admitted to the emergency department of Gachon University Gil Medical Center, a tertiary hospital, and hospitalized between July 1, 2018 and December 31, 2020 were registered for this study. Of these patients, 278 patients who underwent CTA were enrolled in our study.
-
Results
- VTE was found in 120 of the 7,634 patients (1.57%), and the positive diagnosis rate of the 278 patients who underwent CTA was 43.2% (120 of 278). The incidence of VTE was statistically significantly higher among those with severe head and neck injuries (Abbreviated Injury Scale, 3–5) than among those with nonsevere head and neck injuries (Abbreviated Injury Scale, 0–2; P=0.038). In a subgroup analysis, the severe and nonsevere head and neck injury groups showed statistically significant differences in known independent risk factors for VTE. In logistic regression analysis, the adjusted odds ratio of severe head and neck injury (Abbreviated Injury Scale, 3–5) for VTE was 1.891 (95% confidence interval, 1.043–3.430).
-
Conclusions
- Trauma patients with severe head and neck injuries are more susceptible to VTE than those with nonsevere head and neck injuries. Thus, physicians must consider CTA as a priority for the diagnosis of VTE in trauma patients with severe head and neck injuries who show VTE-associated symptoms.
- Venous thromboembolism (VTE), which clinically manifests as pulmonary thromboembolism (PTE) and deep vein thrombosis (DVT), is a significant cause of posttraumatic mortality and morbidity [1–3]. Because trauma is a risk factor for VTE, prompt diagnosis of VTE is important for patients admitted due to trauma [4].
- The overall incidence of VTE among trauma inpatients ranges widely from 0.36% to 1.8%, depending on aspects of study design such as the population and the nature of patients’ injuries [1,5–7]. VTE can be diagnosed using ultrasonography, magnetic resonance imaging, and computed tomography (CT) [8]. Currently, computed tomographic angiography (CTA) is the modality of choice for VTE diagnosis owing to its high sensitivity and specificity (90% and 95%, respectively) [9–11]. Despite its usefulness, CTA is expensive and poses a risk of contrast-induced allergic reactions or nephropathy, and a report has suggested that it can cause delayed radiation-induced solid tumors. For these reasons, routine evaluation using CTA is a difficult decision for physicians [12–14]. Some rule-out criteria can help differentiate VTE. It has been reported that patients who meet all eight criteria in the pulmonary embolism rule-out criteria rule (e.g., age >50 years, recent trauma or surgery) do not require further evaluation, including a D-dimer test, for a differential diagnosis of PTE [8,15]. Furthermore, the YEARS diagnostic algorithm can significantly reduce the use of CT for the diagnosis of PTE in patients suspected to have acute PTE using the D-dimer level and three YEARS items (clinical signs of DVT, hemoptysis, PTE the most likely diagnosis) [16]. Wells’ criteria (clinical signs of DVT, 3 points, heart rate >100 beats/min, recent surgery or immobilization; previous PTE or DVT, 1.5 points, hemoptysis, malignancy; alterative diagnosis less likely than PTE, 1 point) are known to reliably exclude DVT based on a score of below 1 [17]. However, the fact that trauma patients often have VTE risk factors, such as the pathologic effects of trauma and a history of surgery, hinders the application of the above criteria for diagnostic purposes [4]. VTE prediction models can be applied to trauma patients. The Trauma Embolic Scoring System and Greenfield risk assessment profile can be used as appropriate calibration tools for predicting VTE in severely injured trauma patients. The Trauma Embolic Scoring System can predict VTE based on trauma patients’ Injury Severity Score (ISS), age, use of mechanical ventilation, obesity status, and lower limb injuries [18]. The risk assessment profile can predict the likelihood of PTE by considering underlying conditions, iatrogenic factors, Abbreviated Injury Scale (AIS) score, and the Glasgow Coma Scale score [19]. However, although these prediction models can be used for prediction, they are inappropriate as VTE rule-out criteria to reduce the use of CTA. Furthermore, related research on trauma patients in Korea is scarce.
- The aim of this study was to shed light on the epidemiology of trauma inpatients with VTE symptoms diagnosed based on CTA and to compare VTE-positive and VTE-negative patients to identify the factors associated with VTE, thereby ultimately assisting in the decision to perform CTA.
INTRODUCTION
- Ethical statements
- The study protocol was approved by the Institutional Review Board of Gachon University Gil Medical Center (No. GBIRB2021-230). The requirement for informed consent was waived.
- Study design
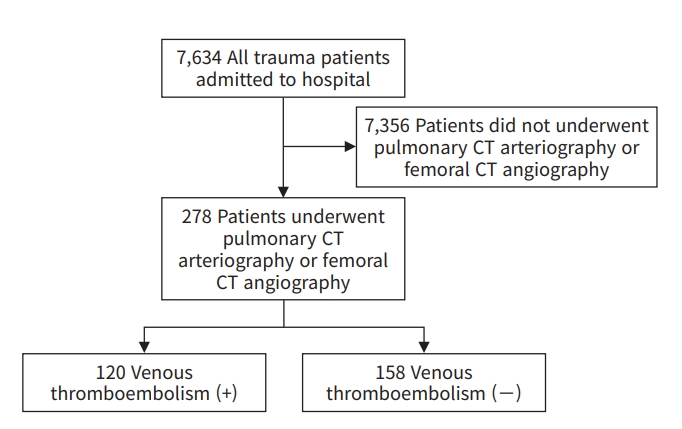
- For this retrospective study, the patient identifications of 7,634 patients admitted to the emergency department of Gachon University Gil Medical Center, a tertiary hospital, and hospitalized between July 1, 2018 and December 31, 2020 were acquired from the Korean Trauma Data Bank (KTDB). These patient identifications were entered into the picture archiving communications system to determine whether CTA was performed. A total of 278 patients underwent CTA, and these patients were enrolled in our study (Fig 1). Information regarding patient epidemiology was selected in consideration of the factors associated with VTE reported in previous studies [1–3,20–22]. From the KTDB, we obtained the following information about patients who underwent CTA: age, sex, ISS (mild and moderate, <16; severe, 16–24; critical, >24), AIS (head and neck, face, chest, abdomen, extremities, and pelvis), admission to the intensive care unit (ICU), and length of ICU stay (days). An electronic medical record review was performed to confirm that CTA was performed on patients suspected of having VTE based on the attending physician’s clinical decision regarding symptoms such as dyspnea; chest pain; hypoxemia; low oxygen saturation upon monitoring; high oxygen demand; swelling, pain, erythema of the lower extremities; and elevated D-dimer levels. Information regarding the use of a mechanical ventilator, length of mechanical ventilation (days), pharmacologic prevention of VTE, use of intravenous (IV) tranexamic acid, major surgery, pelvic fracture, lower extremity fracture, spinal cord injury, and D-dimer levels were obtained from the electronic medical record.
- Definitions
- In this study, an ISS score below 16 was considered to indicate a mild or moderate injury, a score of 16 to 24 was considered indicative of a severe injury, and a score 25 or higher was considered to indicate a critical injury [23–25]. The AIS uses a scale from 1 to 5, with 1 for minor, 2 for moderate, 3 for severe (not life-threatening), 4 for severe (life-threatening), and 5 for critical (survival uncertain) [26]. In this study, an AIS score of 0 to 2 was defined as indicating a nonsevere injury (no to mild injury), and AIS scores of 3 to 5 were defined as indicating a severe injury (severe to critical injury). The tertiary hospital used enoxaparin (40 mg or 60 mg, administered subcutaneously) as the standard protocol for the pharmacologic prevention of VTE in trauma patients. The hospital used IV tranexamic acid as a hemostatic agent to prevent or manage early excessive bleeding.
- Statistical analysis
- Data were statistically analyzed using IBM SPSS ver. 24.0 (IBM Corp., Armonk, NY, USA). A univariate analysis was conducted for noncontinuous variables using the chi-square test, and non-normally distributed continuous variables were analyzed using the Mann-Whitney U-test. The factors associated with VTE onset were identified using logistic regression analysis. A P-value lower than 0.05 was considered to indicate statistical significance.
METHODS
- A total of 7,634 trauma patients were registered in the KTDB from July 1, 2018 to December 31, 2020. After excluding 7,356 patients who did not undergo pulmonary and/or femoral CTA, 278 patients were included in the final analysis (Fig. 1). Of 7,634 patients, 120 developed VTE, corresponding to an incidence rate of 1.57% (120 of 7,634), and the positive diagnosis rate of the 278 patients who underwent CTA was 43.2% (120 of 278) (Fig. 1).
- The clinical characteristics of the VTE-positive and VTE-negative groups, as determined using CTA, were compared. The incidence of VTE was significantly higher among those with severe head and neck injuries (AIS, 3–5) than among those with nonsevere head and neck injuries (AIS, 0–2; P=0.038). There was no statistically significant difference in age between the VTE-positive group (median, 66.5 years; interquartile range, 52.3–78.0 years), and the VTE-negative group (median, 63.6 years; interquartile range, 43.0–80.3). The two groups also did not show statistically significant differences in sex, ISS, AIS (chest, abdomen, extremities, and pelvis), pharmacologic prevention of VTE, use of IV tranexamic acid, major surgery, pelvic fracture, lower extremity fracture, spinal cord injury, admission to the ICU, length of ICU stay (days), use of a mechanical ventilator, length of mechanical ventilation (days), and D-dimer levels. Only two and three patients in the severe injury AIS group (3–5) had AIS (face) and AIS (other) scores, respectively, so those parameters were excluded from the results (Table 1).
- Subgroup analyses were performed for head and neck injuries, which showed a statistically significant difference between the two groups. The severe head and neck injury and nonsevere head and neck injury groups showed statistically significant differences in ISS (P<0.001), use of IV tranexamic acid (P<0.001), lower extremity fracture (P<0.001), spinal cord injury (P<0.001), admission to the ICU (P<0.001), length of ICU stay (days; P<0.001), use of a mechanical ventilator (P<0.001), and length of mechanical ventilation (days; P=0.018) (Table 2).
- To analyze the factors associated with VTE onset, logistic regression analysis was performed with age, sex, head and neck injury, extremities or pelvis injury, and ISS. The adjusted odds ratio for VTE was 1.891 (95% confidence interval, 1.043–3.430) in patients with severe head and neck injuries (AIS, 3–5) (Table 3).
RESULTS
- Even though posttraumatic VTE is an important complication considering its mortality and morbidity, the epidemiology of VTE in trauma patients in Korea is not well known. In a large-scale German study, Paffrath et al. [1] reported that the overall incidence of VTE in inpatients with trauma was 1.8% (146 of 7,937). In their study of 450,000 trauma patients in the United States, Knudson et al. [5] reported a VTE incidence of 0.36% (1,602 of 450,375). Wong et al. [6] found that the incidence of posttraumatic VTE in an Asian population was 0.39% (34 of 8,615) based on a Singaporean database. Boo et al. [7] reported an overall incidence of VTE of 0.87% (82 of 9,472) among Korean trauma patients in their 2021 study. The overall incidence of VTE among trauma inpatients found in this study was 1.57% (120 of 7,634), which is similar to other results. Conventionally, VTE incidence is known to be lower in Asian populations than in Western populations [27]. Based on our and previous study findings, there seems to be no marked difference in VTE incidence among trauma patients in different populations.
- Yumoto et al. [28] reported a VTE diagnosis rate of 32% (65 of 204) among patients with symptoms of posttraumatic VTE based on CT performed at the physician’s discretion. In our study, the rate was 43.2% (120 of 278). Considering these diagnosis rates, the diagnosis should be made aggressively in trauma patients with VTE-associated symptoms.
- The incidence of VTE among patients with head injuries has been extensively studied. Van Gent et al. [29] reported that VTE incidence was higher among patients with isolated traumatic brain injuries who had higher mean head-AIS scores. Knudson et al. [5] stated that a head-AIS score of 3 or higher was a significant independent risk factor for VTE. Our results showed that the VTE rate was higher among patients with severe head and neck injuries (AIS, 3–5) than in patients with no to moderate head and neck injuries (AIS, 0–2) (Table 1). Furthermore, logistic regression analysis revealed that the adjusted odds ratio for VTE was 1.891 (95% confidence interval, 1.043–3.430) in the severe head and neck injury group (AIS, 3–5) (Table 3). Regarding these results, Nekludov et al. [30] reported that elevated levels of interleukin 6 are observed in the blood samples and cerebrospinal fluid samples of patients with isolated traumatic brain injuries and that interleukin 6 may be associated with thrombus generation by increasing the release of acute-phase reactants such as fibrinogen. Mackman [31] argued that after an injury, increased tissue factor release activates the extrinsic clotting cascade. It seems that this mechanism underlies the increased incidence of VTE among patients with traumatic brain injury. Knudson et al. [5] found that lower extremity fracture (AIS ≥3), head injury (AIS ≥3), and major operative procedures were independent risk factors for VTE, and Yumoto et al. [28] reported that a higher risk of ISS score, mechanical ventilation, and longer length of ICU stay were risk factors for VTE. Myers et al. [22] also stated that the use of tranexamic acid was an independent risk factor for VTE. These risk factors are equivalent to the characteristics of patients with severe head and neck injuries obtained through the subgroup analysis in our study. Our subgroup analysis showed that the severe and nonsevere head and neck injury groups differed significantly in the ISS score, use of IV tranexamic acid, lower extremity fracture, spinal cord injury, admission to the ICU, length of ICU stay, use of mechanical ventilator, and length of mechanical ventilation (Table 2). It is speculated that the incidence of VTE may be higher among patients with severe head and neck injuries because these patients already have various VTE risk factors. Byrne et al. [32] reported that early pharmacologic VTE prophylaxis can significantly reduce the incidence of VTE in patients with traumatic brain injury and that pharmacologic VTE prophylaxis can be initiated stably within 72 hours for patients who show stable intracranial hemorrhage on repeated head CT examinations. As patients with head and neck injuries are highly likely to develop VTE, early pharmacologic prophylaxis should be administered to these patients.
- In this study, we attempted to analyze epidemiological differences among patients with posttraumatic VTE-associated symptoms based on their diagnosis of VTE using CTA. No statistically significant differences were found between VTE-positive and VTE-negative patients in known risk factors for VTE, except for the severity of head and neck injuries. Knudson et al. [5] identified age ≥40 years, lower extremity fracture (AIS ≥3), head injury (AIS ≥3), ≥3 days on ventilation, venous injury, and major operative procedures as independent risk factors for VTE. In our study, the characteristics of the patients who underwent CTA were as follows: the median of total age, 64.5 years (interquartile range, 47.5–80.0 years); major surgery, 81.3% (226 of 278); lower extremity fracture, 64.0% (178 of 278); the median of the length of mechanical ventilation, 4.0 days (interquartile range, 1.0–7.0 days). This shows that patients who underwent CTA had the abovementioned risk factors for VTE. The attending physician probably considered CTA for patients at high risk of VTE, such as immobilized patients undergoing major orthopedic surgery. Therefore, patients with similar epidemiological characteristics were enrolled in this study. Hence, the lack of a statistically significant difference in the known VTE risk factors between the VTE-positive and VTE-negative groups, with the exception of head and neck injury severity, may have been because CTA was performed on symptomatic patients who already had VTE risk factors. This study is significant in that it sheds light on the need to consider CTA for VTE diagnosis in trauma inpatients with severe head and neck injuries who have VTE risk factors and show relevant symptoms.
- D-dimer is known to be a useful parameter for avoiding unnecessary tests, such as CT, by ruling out VTE based on its high negative predictive value [28,33,34]. However, trauma patients have elevated D-dimer levels due to the pathological process of trauma itself [21]. Matsumoto et al. [35] reported that there were no significant differences in the D-dimer level among patients with spinal cord injuries, which are considered severe traumatic injuries, according to their DVT diagnosis results. In our study, the median D-dimer level was 6,338 ng/mL (interquartile range, 4,004–12,950 ng/mL) in the VTE-positive group and 6,640 ng/mL (interquartile range, 2,408–14,806 ng/mL) in the VTE-negative group, showing that these patients had markedly elevated D-dimer levels relative to the normal values, even considering age-adjusted D-dimer cutoff values. Two patients in the negative group had values below the cutoff value, and the negative group had a higher median value (Table 1). Thus, according to our results, the D-dimer level is inadequate as a single screening factor for VTE detection in trauma patients. Yumoto et al. [28] reported that VTE occurred within a median of 10 days from hospitalization in trauma patients and that the D-dimer level after about 10 days of admission had moderate accuracy as a predictor of VTE onset. We observed during our data collection that D-dimer testing was sometimes not performed and serial examinations were rarely performed in trauma patients. Serial D-dimer levels from admission to 10 days later may be meaningful for trauma patients anticipated to have a prolonged hospital stay.
- This study has a few limitations. First, this was a retrospective study, and the final analysis was performed on patients who underwent CTA. The decision to perform CTA was made at the discretion of the clinician based on patients’ presentation of signs or symptoms known to be risk factors for VTE. However, this leaves our study vulnerable to selection bias, as patients who developed VTE without undergoing CTA were not included. Thus, prospective studies with a strict study design including screening tests such as venous ultrasound for an early diagnosis of VTE are required.
- Second, this was a single-center study. While data from 7,634 patients registered in the KTDB were reviewed, only 278 patients were enrolled in the study due to the rare nature of the disease. Thus, a large-scale multicenter study is needed to produce more statistically significant results.
- In conclusion, there is no marked difference in the incidence of VTE among inpatients with trauma across populations. Trauma patients with severe head and neck injuries were found to be more susceptible to VTE than those with nonsevere head and neck injuries. Thus, attending physicians must consider CTA as a priority for the diagnosis of VTE in trauma patients with severe head and neck injuries who show VTE-associated symptoms. Aggressive and routine measurements for VTE can effectively reduce the incidence of clinically significant VTE and enable an early diagnosis of VTE in trauma patients. Attending physicians should be aware of and predict VTE risk factors and symptoms in trauma patients, and healthcare facilities should develop a VTE prevention and diagnosis protocol for this group of patients.
DISCUSSION
-
Ethical statements
The study protocol was approved by the Institutional Review Board of Gachon University Gil Medical Center (No. GBIRB2021-230). The requirement for informed consent was waived.
-
Conflicts of interest
The authors have no conflicts of interest to declare.
-
Funding
None.
-
Author contributions
Conceptualization: all authors; Data curation: all authors; Formal analysis: HSP, SYH, WSC, JSC, JYC; Methodology: all authors; Project administration: HSP, SYH, WSC; Visualization: HSP, SYH, WSC,J SC; Writing–original draft: HSP, WSC; Writing–review & editing: HSP, SYH, WSC, JHJ, JYC.
All authors read and approved the final manuscript.
ARTICLE INFORMATION
| Characteristic | Severe head and neck injurya) | Nonsevere head and neck injuryb) | P-value |
|---|---|---|---|
| Total | 58 (100) | 220 (100) | |
| VTE | 0.038 | ||
| – | 26 (44.8) | 132 (60.0) | |
| + | 32 (55.2) | 88 (40.0) | |
| Pulmonary thromboembolism | 16 (50.0) | 44 (50.0) | |
| Deep vein thrombosis | 28 (87.5) | 68 (77.3) | |
| ISS of patients | <0.001 | ||
| Mild and moderate (<16) | 10 (17.2) | 177 (80.5) | |
| Severe (16–24) | 29 (50.0) | 32 (14.5) | |
| Critical (>24) | 19 (32.8) | 11 (5.0) | |
| Independent risk factor | |||
| Chemical prevention of VTE | 15 (25.9) | 79 (35.9) | 0.150 |
| Use of IV tranexamic acid | 35 (60.3) | 34 (15.5) | <0.001 |
| Major surgery | 47 (81.0) | 179 (81.4) | 0.954 |
| Pelvic fracture | 10 (17.2) | 32 (14.5) | 0.610 |
| Lower extremity fracture | 21 (36.2) | 157 (71.4) | <0.001 |
| Spinal cord injury | 18 (31.0) | 15 (6.8) | <0.001 |
| Admission to ICU | 50 (86.2) | 84 (38.2) | <0.001 |
| Length of ICU stay (day) (n=134) | 50 (37.3) | 84 (62.7) | <0.001 |
| Median (IQR) | 9.5 (4.0–14.0) | 3.0 (1.0–6.0) | |
| Use of mechanical ventilator | 37 (63.8) | 39 (17.7) | <0.001 |
| Length of mechanical ventilation (day) (n=76) | 37 (48.7) | 39 (51.3) | 0.018 |
| Median (IQR) | 5.0 (3.0–8.0) | 2.0 (0.0–6.0) | |
| D-dimer (n=204) | 0.437 | ||
| Elevation | 47 (100) | 155 (98.7) | |
| Nonelevation | 0 | 2 (1.3) | |
| Median (IQR) | 7,900 | 6,100 | 0.292 |
| (4,760–1,4020) | (3,417–14,556) |
- 1. Paffrath T, Wafaisade A, Lefering R, et al. Venous thromboembolism after severe trauma: incidence, risk factors and outcome. Injury 2010;41:97–101. ArticlePubMed
- 2. Geerts WH, Code KI, Jay RM, Chen E, Szalai JP. A prospective study of venous thromboembolism after major trauma. N Engl J Med 1994;331:1601–6. ArticlePubMed
- 3. Thorson CM, Ryan ML, Van Haren RM, et al. Venous thromboembolism after trauma: a never event. Crit Care Med 2012;40:2967–73. ArticlePubMed
- 4. Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest 2008;133(6 Suppl):381S–453S. ArticlePubMed
- 5. Knudson MM, Ikossi DG, Khaw L, Morabito D, Speetzen LS. Thromboembolism after trauma: an analysis of 1602 episodes from the American College of Surgeons National Trauma Data Bank. Ann Surg 2004;240:490–8. ArticlePubMedPMC
- 6. Wong TH, Koh MP, Ng J. Symptomatic venous thromboembolism in Asian major trauma patients: incidence, presentation and risk factors. Eur J Trauma Emerg Surg 2013;39:495–500. ArticlePubMedPDF
- 7. Boo S, Oh H, Hwang K, Jung K, Moon J. Venous thromboembolism in a single Korean trauma center: incidence, risk factors, and assessing the validity of VTE diagnostic tools. Yonsei Med J 2021;62:520–7. ArticlePubMedPMCPDF
- 8. Singh B, Mommer SK, Erwin PJ, Mascarenhas SS, Parsaik AK. Pulmonary embolism rule-out criteria (PERC) in pulmonary embolism: revisited: a systematic review and meta-analysis. Emerg Med J 2013;30:701–6. ArticlePubMed
- 9. Mamlouk MD, vanSonnenberg E, Gosalia R, et al. Pulmonary embolism at CT angiography: implications for appropriateness, cost, and radiation exposure in 2003 patients. Radiology 2010;256:625–32. ArticlePubMed
- 10. Stein PD, Fowler SE, Goodman LR, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med 2006;354:2317–27. ArticlePubMed
- 11. Thomas SM, Goodacre SW, Sampson FC, van Beek EJ. Diagnostic value of CT for deep vein thrombosis: results of a systematic review and meta-analysis. Clin Radiol 2008;63:299–304. ArticlePubMed
- 12. Cochran ST, Bomyea K, Sayre JW. Trends in adverse events after IV administration of contrast media. AJR Am J Roentgenol 2001;176:1385–8. ArticlePubMed
- 13. Mitchell AM, Kline JA. Contrast nephropathy following computed tomography angiography of the chest for pulmonary embolism in the emergency department. J Thromb Haemost 2007;5:50–4. ArticlePubMed
- 14. Verdun FR, Bochud F, Gundinchet F, Aroua A, Schnyder P, Meuli R. Quality initiatives radiation risk: what you should know to tell your patient. Radiographics 2008;28:1807–16. ArticlePubMed
- 15. Hugli O, Righini M, Le Gal G, et al. The pulmonary embolism rule-out criteria (PERC) rule does not safely exclude pulmonary embolism. J Thromb Haemost 2011;9:300–4. ArticlePubMed
- 16. van der Hulle T, Cheung WY, Kooij S, et al. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet 2017;390:289–97. ArticlePubMed
- 17. Modi S, Deisler R, Gozel K, et al. Wells criteria for DVT is a reliable clinical tool to assess the risk of deep venous thrombosis in trauma patients. World J Emerg Surg 2016;11:24. ArticlePubMedPMC
- 18. Ho KM, Rao S, Rittenhouse KJ, Rogers FB. Use of the Trauma Embolic Scoring System (TESS) to predict symptomatic deep vein thrombosis and fatal and non-fatal pulmonary embolism in severely injured patients. Anaesth Intensive Care 2014;42:709–14. ArticlePubMedPDF
- 19. Gearhart MM, Luchette FA, Proctor MC, et al. The risk assessment profile score identifies trauma patients at risk for deep vein thrombosis. Surgery 2000;128:631–40. ArticlePubMed
- 20. Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation 2003;107(23 Suppl 1):I9–16. ArticlePubMed
- 21. Palta S, Saroa R, Palta A. Overview of the coagulation system. Indian J Anaesth 2014;58:515–23. ArticlePubMedPMC
- 22. Myers SP, Kutcher ME, Rosengart MR, et al. Tranexamic acid administration is associated with an increased risk of posttraumatic venous thromboembolism. J Trauma Acute Care Surg 2019;86:20–7. ArticlePubMed
- 23. Stevenson M, Segui-Gomez M, Lescohier I, Di Scala C, McDonald-Smith G. An overview of the injury severity score and the new injury severity score. Inj Prev 2001;7:10–3. ArticlePubMedPMC
- 24. Jung K, Lee JC, Kim J. Injury severity scoring system for trauma patients and trauma outcomes research in Korea. J Acute Care Surg 2016;6:11–7. Article
- 25. Baker SP, O’Neill B. The injury severity score: an update. J Trauma 1976;16:882–5. ArticlePubMed
- 26. Greenspan L, McLellan BA, Greig H. Abbreviated Injury Scale and Injury Severity Score: a scoring chart. J Trauma 1985;25:60–4. ArticlePubMed
- 27. Lee LH, Gallus A, Jindal R, Wang C, Wu CC. Incidence of venous thromboembolism in Asian populations: a systematic review. Thromb Haemost 2017;117:2243–60. ArticlePubMed
- 28. Yumoto T, Naito H, Yamakawa Y, Iida A, Tsukahara K, Nakao A. Venous thromboembolism in major trauma patients: a single-center retrospective cohort study of the epidemiology and utility of D-dimer for screening. Acute Med Surg 2017;4:394–400. ArticlePubMedPMCPDF
- 29. Van Gent JM, Bandle J, Calvo RY, et al. Isolated traumatic brain injury and venous thromboembolism. J Trauma Acute Care Surg 2014;77:238–42. ArticlePubMed
- 30. Nekludov M, Antovic J, Bredbacka S, Blomback M. Coagulation abnormalities associated with severe isolated traumatic brain injury: cerebral arterio-venous differences in coagulation and inflammatory markers. J Neurotrauma 2007;24:174–80. ArticlePubMed
- 31. Mackman N. Tissue-specific hemostasis: role of tissue factor. J Thromb Haemost 2008;6:303–5. ArticlePubMed
- 32. Byrne JP, Mason SA, Gomez D, et al. Timing of pharmacologic venous thromboembolism prophylaxis in severe traumatic brain injury: a propensity-matched cohort study. J Am Coll Surg 2016;223:621–31. ArticlePubMed
- 33. Niikura T, Sakai Y, Lee SY, et al. D-dimer levels to screen for venous thromboembolism in patients with fractures caused by high-energy injuries. J Orthop Sci 2015;20:682–8. ArticlePubMed
- 34. Owings JT, Gosselin RC, Anderson JT, Battistella FD, Bagley M, Larkin EC. Practical utility of the D-dimer assay for excluding thromboembolism in severely injured trauma patients. J Trauma 2001;51:425–30. ArticlePubMed
- 35. Matsumoto S, Suda K, Iimoto S, et al. Prospective study of deep vein thrombosis in patients with spinal cord injury not receiving anticoagulant therapy. Spinal Cord 2015;53(4):306–9. ArticlePubMedPDF
REFERENCES
Figure & Data
References
Citations

 KST
KST

 PubReader
PubReader ePub Link
ePub Link Cite
Cite


