The effect of neuropathic pain on quality of life, depression levels, and sleep quality in patients with combat-related extremity injuries
Article information
Abstract
Purpose
There is limited research on the effects of neuropathic pain (NP) on quality of life, depression levels, and sleep quality in patients with combat-related extremity injuries. This study evaluated whether patients with combat-related extremity injuries with and without NP had differences in quality of life, sleep quality, and depression levels.
Methods
A total of 98 patients with combat-related extremity injuries, 52 with NP and 46 without, were included in this cross-sectional study. The presence of NP was determined using the Leeds Assessment of Neuropathic Symptoms and Signs questionnaire. The outcome measures were a visual analogue scale (VAS), the 36-Item Short Form Survey, the Beck Depression Inventory, and the Pittsburgh Sleep Quality Index (PSQI).
Results
The VAS subparameter scores for pain (all P<0.05), PSQI sleep duration subscale scores (P=0.025), PSQI sleep disturbance subscale scores (P=0.016), and PSQI total scores (P=0.020) were significantly higher in patients with NP than those without. Logistic regression analysis showed that VAS scores of 5 and above for average pain during the previous 4 weeks contributed independently to the prediction of NP.
Conclusions
Patients with combat-related extremity injuries with NP had more pain and poorer sleep quality than those without NP. Sleep quality should be evaluated as part of the diagnostic work-up in patients with combat-related extremity injury with NP, and interventions to improve sleep quality may help manage NP in this patient group.
INTRODUCTION
Modern combat body armor is designed to protect the abdomen and chest, but provides limited protection for the limbs. Fragmentation and blast injuries cause multidimensional and complex wounding patterns. Multiple injuries involving neural destruction, extensive soft tissue defects and bone destruction are common in the extremities [1–3].
Chronic pain is common among military veterans and is associated with lower quality of life (QoL), functional disability, and psychological symptoms [4,5]. It has also been suggested that veterans with chronic pain might have increased risks for posttraumatic stress disorder, depression, and anxiety [6,7]. However, most of the previous studies have considered chronic pain as a whole, and limited research exists on the association of neuropathic pain (NP) with QoL and psychological comorbidity [8].
NP is a direct result of a disease or lesion affecting the somatosensory system [9]. Typical clinical features of NP include spontaneous pain, paroxysmal pain, burning, ice-cold sensations, electrical shocks, or abnormally intense responses to nonpainful (allodynia) or painful (hyperalgesia) stimuli [10].
Studies with civilian subjects have shown that patients with NP symptoms have higher levels of depression and anxiety, and worse health-related QoL than the general population [11–13]. There is, however, limited research on the extent to which NP influences QoL, levels of depression, and sleep quality in patients with combat-related extremity injuries. Therefore, this study investigated differences in QoL, depression level, and sleep quality among such patients with and without NP.
METHODS
Participants and study design
This was a cross-sectional study conducted in the Orthopedic Rehabilitation Unit of Gaziler Physical Medicine and Rehabilitation, Training and Research Hospital (Ankara, Turkey), a tertiary hospital, from July 2020 to November 2021. A sample size calculation was performed using G*Power ver. 3.1 (Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany; http://www.gpower.hhu.de/). In order to detect a difference at 5% type 1 error level with 90% power for an effect size of 0.62 [14], a minimum of 46 patients was required for each group. A total of 98 patients with combat-related extremity injuries, 52 with NP and 46 without, were included. The inclusion criteria were previous history of combat-related extremity injury, age of 18 to 65 years, and time after injury ≥3 months. Exclusion criteria were known rheumatic disease, neurologic, or endocrine disorders; previous history of cervical or lumbosacral radiculopathy; excessive alcohol consumption; and vitamin B12 deficiency. The study was approved by the Ethics Committee of Ankara City Hospital (No. E1-20-838). The study was also registered with the ClinicalTrials.gov database (No. NCT04674631). Written informed consent was obtained from all patients.
Demographic and clinical variables
The subjects’ demographic and clinical features including age, weight, height, education, occupation, side of injury, type of injury, and duration of pain were noted. A 10-cm visual analogue scale (VAS) was used to measure the severity of pain [15]. The presence of NP was established using the Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) questionnaire [16]. The Turkish adaptation of LANSS was developed by Yucel et al. [17] and has been shown to be a valid scale. The 98 patients were divided into two groups based on the absence or presence of NP. The NP group included 52 patients with scores of 12 or more on the LANSS, while the 46 patients with scores less than 12 were the group without NP.
The impact of pain on health-related QoL was assessed using the 36-Item Short Form Survey (SF-36). SF-36 is a common tool for evaluating QoL, and includes 36 items in eight separate scales (physical role, physical functioning, general health, bodily pain, social functioning vitality, mental health, and emotional role). The subscales are scored between 0 and 100, and better QoL is indicated by higher scores [18]. The Turkish version of the SF-36 has been found to be valid and reliable [18].
The Beck Depression Inventory (BDI) was used to evaluate the depression status of the patients. BDI measures symptoms and attitudes characteristic of depression [19]. The 21-item scale is scored between 0 and 63. The Turkish version of BDI has been shown to be valid and reliable [20]. Scores over 17 indicate clinically significant depression. The full depression scale for this test is minimal, 0 to 9; mild, 10 to 16; moderate, 17 to 29; and severe, 30 to 63 [21].
The patients’ self-reported sleep quality during the previous month was assessed using the Pittsburgh Sleep Quality Index (PSQI) [22]. The index includes 19 items and evaluates seven components of sleep quality (subjective quality of sleep, sleep duration, sleep latency, sleep disturbances, sleep efficiency, daytime dysfunction, and drug use for sleep). The total PSQI score is the sum of scores from the seven components, and ranges from 0 to 21. Scores of 6 or higher indicate poor quality of sleep [23]. The Turkish version of PSQI has been validated [24].
Statistical analysis
The study data were analyzed using IBM SPSS ver. 23.0 (IBM Corp., Armonk, NY, USA). Nonsignificant results from the Kolmogorov-Smirnov test were used to show the normality of data distribution. Continuous variables were presented as mean±standard deviation and categorical variables as percentages (%). The Pearson chi-square test, independent-samples t-test, and Mann-Whitney U-test were used to compare the two groups. Pearson and Spearman correlation analyses were used to evaluate the relationships between LANSS scores and demographic and clinical parameters in patients with NP. A P-value less than 0.05 was considered to indicate statistical significance.
Logistic regression analysis was performed using possible factors identified in previous analyses to predict NP. The independent factors included in the model were age, duration of pain (months), SF-36 pain subscale scores, the PSQI sleep disturbance and sleep duration subscale scores, PSQI total scores, the percentage of the patients with poor sleep quality (PSQI total score, <6 vs. ≥6), VAS during movement scores (<5 vs. ≥5), VAS average pain during the past 4 weeks scores (<5 vs. ≥5), and VAS night pain scores (<5 vs. ≥5). The Hosmer-Lemeshow test was used for model fit. For each variable, 95% confidence intervals and odds ratios were calculated, and the Wald test was performed to test significance.
RESULTS
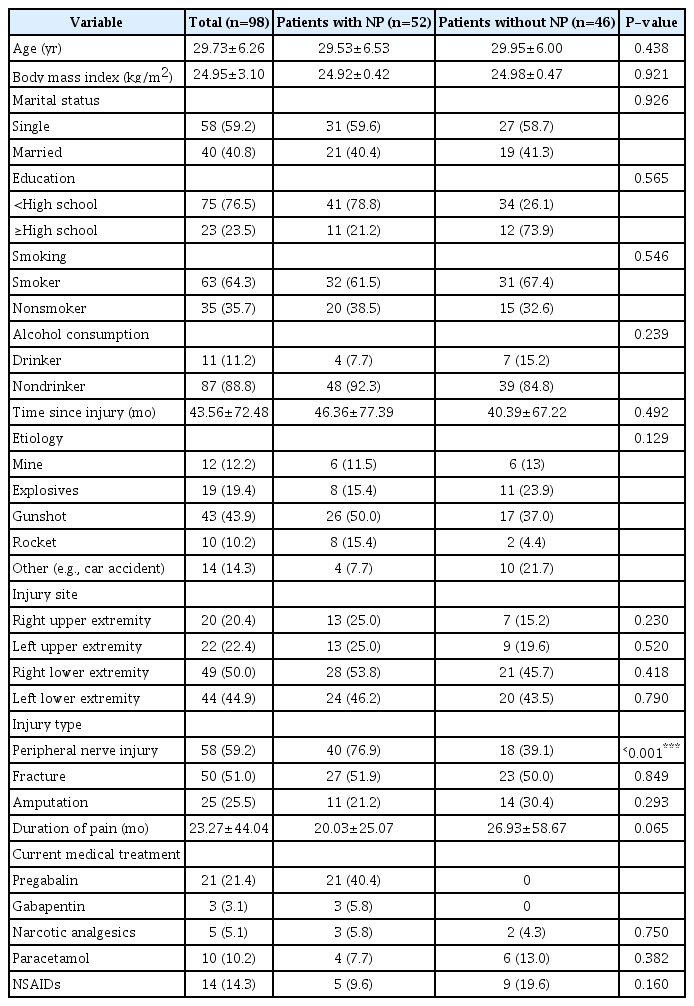
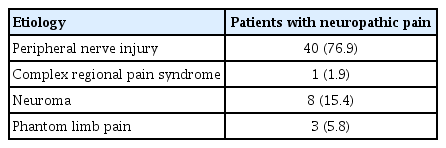
This study included 98 male subjects; 3 to 312 months had elapsed since their injuries in patients with NP and 3 to 264 months in patients without NP. The most common type of injury was gunshot wounds, accounting for 50% of the patients with NP. The frequency of peripheral nerve injuries was higher in the NP group (P<0.001). Demographic variables of the patients are shown in Table 1, and the etiology of NP is presented in Table 2.
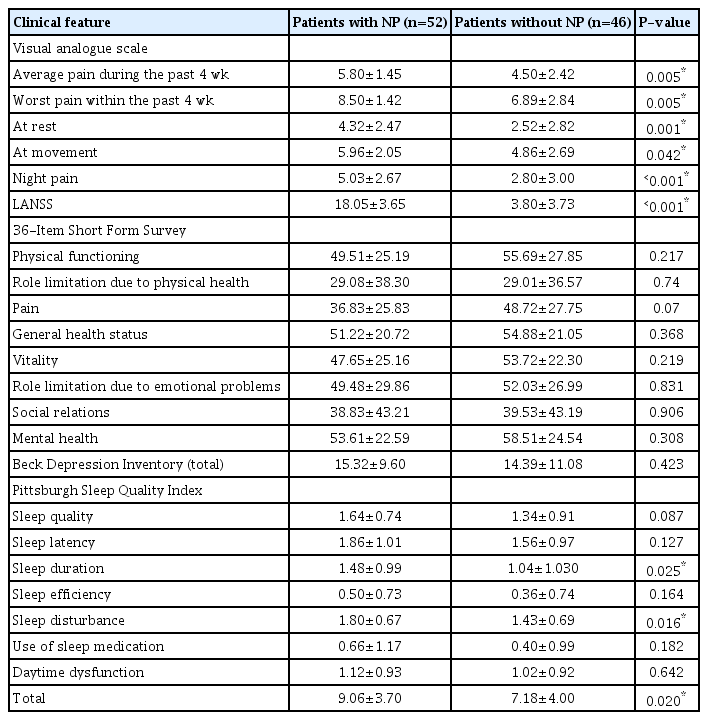
The VAS subparameter scores for pain (all P<0.05), PSQI sleep duration subscale scores (P=0.025), PSQI sleep disturbance subscale scores (P=0.016), and PSQI total scores (P=0.020) were significantly higher in patients with NP than in those without NP (Table 3). Moderate or severe depression was found in 40.4% of the patients with NP and in 32.6% of those without NP. There was no statistically significant difference between the two groups in terms of depression severity (P=0.523). Poor sleep quality was found in 76.0% of the patients with NP and 59.0% of those without NP (P=0.066).
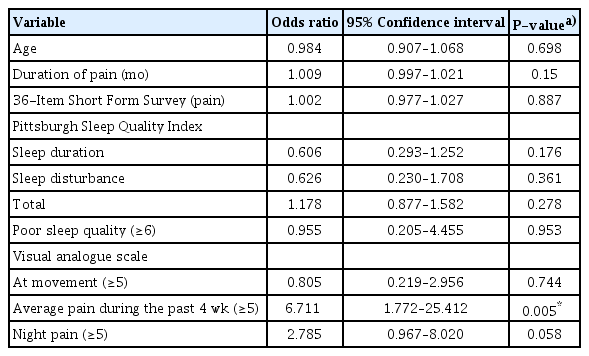
In patients with NP, LANSS scores correlated significantly with VAS during movement (r=0.376, P=0.006), the SF-36 general health subscale (r=–0.326, P=0.022), and the PSQI sleep disturbances subscale (r=0.374, P=0.007) (Table 4). Logistic regression analysis showed that VAS scores of 5 and above for average pain during the previous 4 weeks contributed independently to the prediction of NP (Table 5).
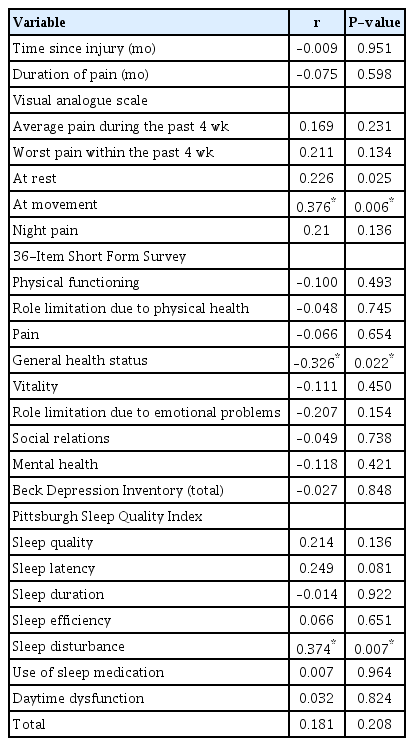
Correlations between LANSS scores and demographic and clinical variables in patients with neuropathic pain
DISCUSSION
Our results showed that patients with combat-related extremity injuries with NP had more pain and poorer sleep quality than those without NP. LANSS scores were also significantly associated with VAS during movement, the SF-36 general health subscale, and the PSQI sleep disturbances subscale in patients with NP.
The release of inflammatory mediators following tissue trauma can lead to primary or secondary peripheral sensitization of nociceptors, making them more sensitive to stimulation and causing a “wind-up” of spinal cord activity. This can cause the continuation of pain and if left untreated, central sensitization can occur, leading to chronic NP with persistent perceptions that last after tissue repair is complete [1].
Our findings showed that all VAS subparameter scores for pain were significantly higher in patients with NP than in those without NP. This finding is broadly consistent with studies showing that patients with mostly neuropathic chronic pain often report more severe pain [13,25]. Moreover, VAS scores of 5 and above for average pain during the previous 4 weeks were shown to independently contribute to the prediction of NP in our study.
A nationwide epidemiological study of the general population in France demonstrated that participants with NP exhibited lower QoL, poorer sleep quality, and more depression and anxiety symptoms than those without NP [11]. In our study, in contrast, no difference was found between the two groups (with and without NP) in terms of QoL and depression, except for sleep quality.
Besides NP, other factors can affect the QoL and depression status of patients with combat-related injuries. Woodruff et al. [26] showed that health-related QoL among service members injured in combat was associated with demographic factors, injury and service experience, and most strongly with present mental health status. Grieger et al. [27] demonstrated that the clinical characteristics of injuries (e.g., mechanism and severity) in battle-injured soldiers were related to the risk of developing depression and postinjury posttraumatic stress disorder, as well as acute and chronic pain. In our study, the etiology of injury was similar in both groups, and we interpret the similarity of the SF-36 scores as reflecting the broad similarity of the demographic variables and severity of depression in the two patient groups.
To the best of the authors' knowledge, only one previous study compared patients with combat-related extremity injuries with and without NP [8]. That study of combat-injured Danish soldiers showed that NP was associated with deterioration of self-rated health and increased psychological distress. The sample size in that study, in which the presence of NP was evaluated with the PainDETECT questionnaire, was smaller than our study and sleep quality was not evaluated [8].
The prevalence of sleep disturbances ranges from 50% to 80% in patients with chronic pain, and the severity of sleep disturbances is associated with pain intensity [28]. Melikoglu et al. [23] showed that 80% of patients with NP had poor sleep quality regardless of the cause of NP. The detected rate of poor sleep quality of 76% in this study for patients with NP is comparable to previous findings in the literature. To our knowledge, no previous studies have compared sleep quality in patients with combat-related extremity injuries with and without NP. As in the related civilian studies [11,23], sleep quality in patients with NP was found to be poorer than in those without NP in our study. Moreover, a correlation was found between LANSS scores and the PSQI sleep disturbances subscale in patients with NP. Although the difference in the percentage of the patients with poor sleep quality between the two groups did not reach statistical significance, the P-value was close to 0.05.
This study has some limitations. The study population and time after injury were heterogeneous. The results of the study cannot be generalized since all the participants were male and from a single center. The uneven number of patients between the two groups is another limitation. Detailed information on injury-related features was not obtained using specific scales such as the Injury Severity Score or Abbreviated Injury Scale. In this cross-sectional study, we also could not evaluate changes in and effects of NP in patients over time. A prospective cohort study is needed to obtain a more complete evaluation of the NP in patients with combat-related extremity injuries. Lastly, the number of patients with amputation in our study was small. We could not compare patients with NP with and without amputation in terms of QoL, depression level, and sleep quality because we did not track amputations. Further studies with more patients with amputation are required to compare patients with NP with and without amputation in terms of QoL, depression level, and sleep quality.
Patients with combat-related extremity injuries with NP had more pain and poorer sleep quality than those without NP. Sleep quality should be evaluated as part of the diagnostic work-up in patients with combat-related extremity injuries with NP, and approaches to boost sleep quality may contribute to the overall management of NP in this patient group. Future studies addressing the limitations of this study are warranted.
Notes
Ethical statements
The study was approved by the Ethics Committee of NAME (No. E1-20-838). The study was also registered with the ClinicalTrials.gov database (No. NCT04674631). Written informed consent was obtained from all patients.
Conflicts of interest
The authors have no conflicts of interest to declare.
Funding
None.
Author contributions
Conceptualization: MÖA, GKK, KA; Data curation: MÖA, GKK, FÖ, YD; Formal analysis: MÖA, GKK, FÖ, YD; Methodology: MÖA, GKK, KA; Writing–original draft: MÖA, YD; Writing–review & editing: all authors.
All authors read and approved the final manuscript.