Articles
- Page Path
- HOME > J Trauma Inj > Volume 36(2); 2023 > Article
-
Original Article
Experience of surgical treatments for abdominal inferior vena cava injuries in a regional trauma center in Korea -
Jin Woo Park, MD1
 , Dong Hun Kim, MD2
, Dong Hun Kim, MD2
-
Journal of Trauma and Injury 2023;36(2):105-113.
DOI: https://doi.org/10.20408/jti.2023.0001
Published online: June 15, 2023
- 1,122 Views
- 66 Download
1Department of Surgery, Dankook University Hospital, Cheonan, Korea
2Division of Trauma Surgery, Department of Surgery, Dankook University College of Medicine, Cheonan, Korea
- Correspondence to Dong Hun Kim, MD Division of Trauma Surgery, Department of Surgery, Dankook University College of Medicine, 119 Dandae-ro, Dongnam-gu, Cheonan 31116, Korea Tel: +82-41-550-3042 Email: saint7331@gmail.com
Copyright © 2023 The Korean Society of Traumatology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Purpose
- Inferior vena cava (IVC) injuries are a rare type of traumatic abdominal injuries that are challenging to treat and have a very high mortality rate. This study described our experience with the surgical treatment of traumatic IVC injuries, and we investigated the demographics, clinical profiles, and surgical outcomes of cases at a regional trauma center.
-
Methods
- Among the 16 patients who were treated for a traumatic IVC injury between January 2014 and March 2022, 14 underwent surgery. The surgical outcomes included overall mortality and 24-hour mortality, and we investigated the factors associated with these surgical outcomes. The 14 patients were divided into two groups according to the location of the IVC injury (retrohepatic IVC or higher vs. subhepatic IVC), and differences between the two groups were analyzed.
-
Results
- A body mass index (BMI) >23.0 kg/m2 (P=0.046), an elevated serum lactate level (P=0.043), and a shorter operation time (P=0.016) were associated with overall mortality. A higher BMI (P=0.050), higher serum lactate level (P=0.004), shorter operation time (P=0.005), and an injury at the retrohepatic IVC or higher level (P=0.031) were associated with 24-hour mortality. Younger age (P=0.028), higher BMI (P=0.005), more acidic pH, higher lactatemia (P=0.012), a higher hemoglobin level (P=0.012), and shorter door-to-operating room time (P=0.028) were associated with injury at the retrohepatic IVC or higher level. Patients with subhepatic IVC injuries had a high rate of direct repair (75.0%) and a significantly lower 24-hour mortality rate (37.5%, P=0.031).
-
Conclusions
- Subhepatic IVC injuries are easy to access and are usually expected to treat with a direct repair method. Injuries at the retrohepatic IVC or higher level are difficult to treat surgically and require a systematic and multidisciplinary treatment strategy.
- Traumatic inferior vena cava (IVC) injuries are rare among traumatic abdominal injuries, accounting for fewer than 5% of penetrating injuries and 0.5% of blunt trauma injuries [1]. However, they are often fatal, with prehospital and in-hospital mortality rates of 30% to 50% and 20% to 66%, respectively [2].
- There are various methods for treating traumatic IVC injuries, including surgery, vascular grafting, and even close monitoring, depending on the injury level and patient stability. Although IVC repair is the mainstay treatment, in cases where repair is challenging because of massive bleeding, difficult access to the injured site, or notable patient instability, ligation is a treatment option [3]. An atriocaval shunt can also be used to repair the retrohepatic IVC segment [4]. This variety of treatment methods illustrates the lack of consensus or guidelines, and survival rates have not significantly improved over time [5].
- Currently, a higher level of injury, blunt trauma, and a lower Glasgow Coma Scale (GCS) score are associated with worse outcomes of traumatic IVC injuries [3,6–8]. However, relatively few cases of IVC injuries have been studied to identify the prognostic factors.
- IVC injuries often require emergency treatment, and it can be difficult to collect data in those urgent situations. Moreover, IVC injuries are rare and information is scarce. In Korea, where the availability of authorized trauma centers is relatively recent, there have been few studies on traumatic IVC injuries, and most are case reports. Therefore, this study aimed to describe our experience with the surgical treatment of traumatic IVC injuries and to investigate the demographics, clinical profiles, and surgical outcomes of cases at a regional trauma center.
INTRODUCTION
- Ethics statements
- This study was reviewed and approved by the Institutional Review Board Dankook University Hospital (No. DKUH 2022-11-034). The requirement for informed consent was waived by the Institutional Review Board because this was a retrospective study and only anonymized data were used.
- Description of participants
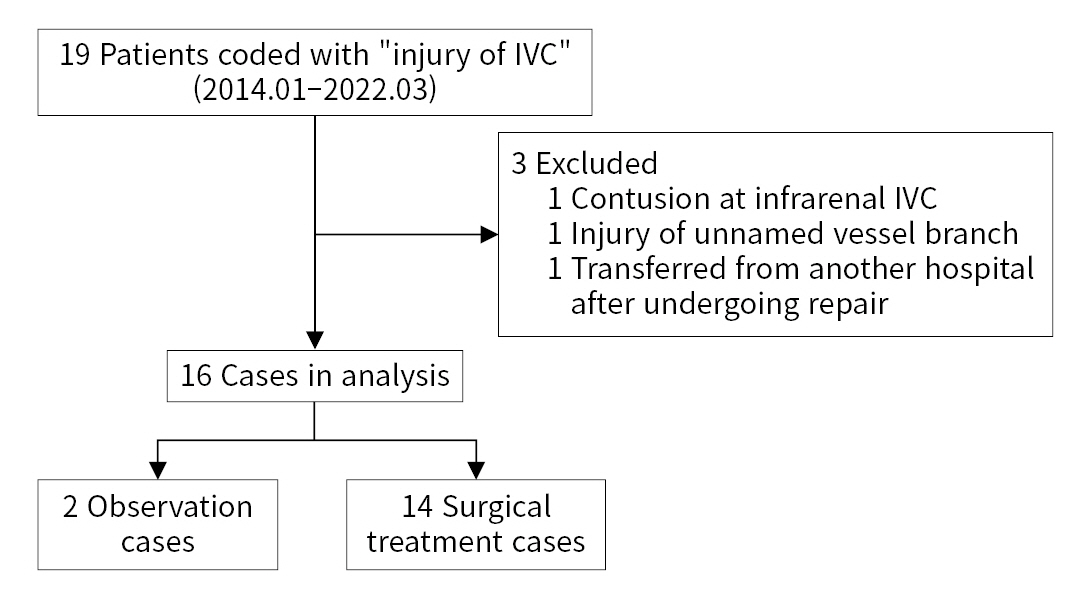
- The medical records of patients admitted to a single regional trauma center for traumatic IVC injuries between January 2014 and March 2022 were retrospectively reviewed. Nineteen patients with IVC injuries were identified. Among them, one patient had an IVC contusion only, one patient had an unnamed vessel branch injured near the IVC, and one patient was transferred from another hospital after already undergoing repair. Thus, 16 patients were enrolled after the three above-mentioned patients were excluded. Of these 16 patients, two underwent medical treatment without surgery. Finally, 14 patients were enrolled for the analysis of surgical outcomes (Fig. 1). The surgical outcomes included overall mortality and 24-hour mortality. We aimed to identify the factors associated with these surgical outcomes and the differences between patients with injuries at the retrohepatic IVC or higher level and those with injuries at the subhepatic IVC level.
- Data collection
- Data regarding patient demographics, injury levels, initial clinical characteristics, initial laboratory values, treatments, and surgical outcomes were collected. The demographics included age, sex, body mass index (BMI), injury mechanism, cause of injury, prehospital time, Injury Severity Score (ISS), and Abbreviated Injury Scale for each body part. Initial clinical characteristics included systolic blood pressure (SBP), heart rate per minute, mean arterial pressure (MAP), and GCS score. Initial laboratory values included pH, partial pressure of arterial oxygen, serum lactate level, hemoglobin, and international normalized ratio. Treatment data included cardiopulmonary resuscitation (CPR) in the emergency room (ER), door-to-operating room (DTO) time, main treatment methods (direct repair, ligation, atriocaval shunt, observation, and others), amount of red blood cells (RBCs) transfused within the first 24 hours, and operation time. “Others” in the main treatment methods included procedures to stop bleeding such as gauze packing, direct manual compression, and resuscitative endovascular balloon occlusion of the aorta.
- If the patient was intubated on arrival, the GCS verbal score was calculated using a linear regression model as follows [9]:
- Derived verbal score = −0.3756 + motor score × (0.5713) + eye score × (0.4233)
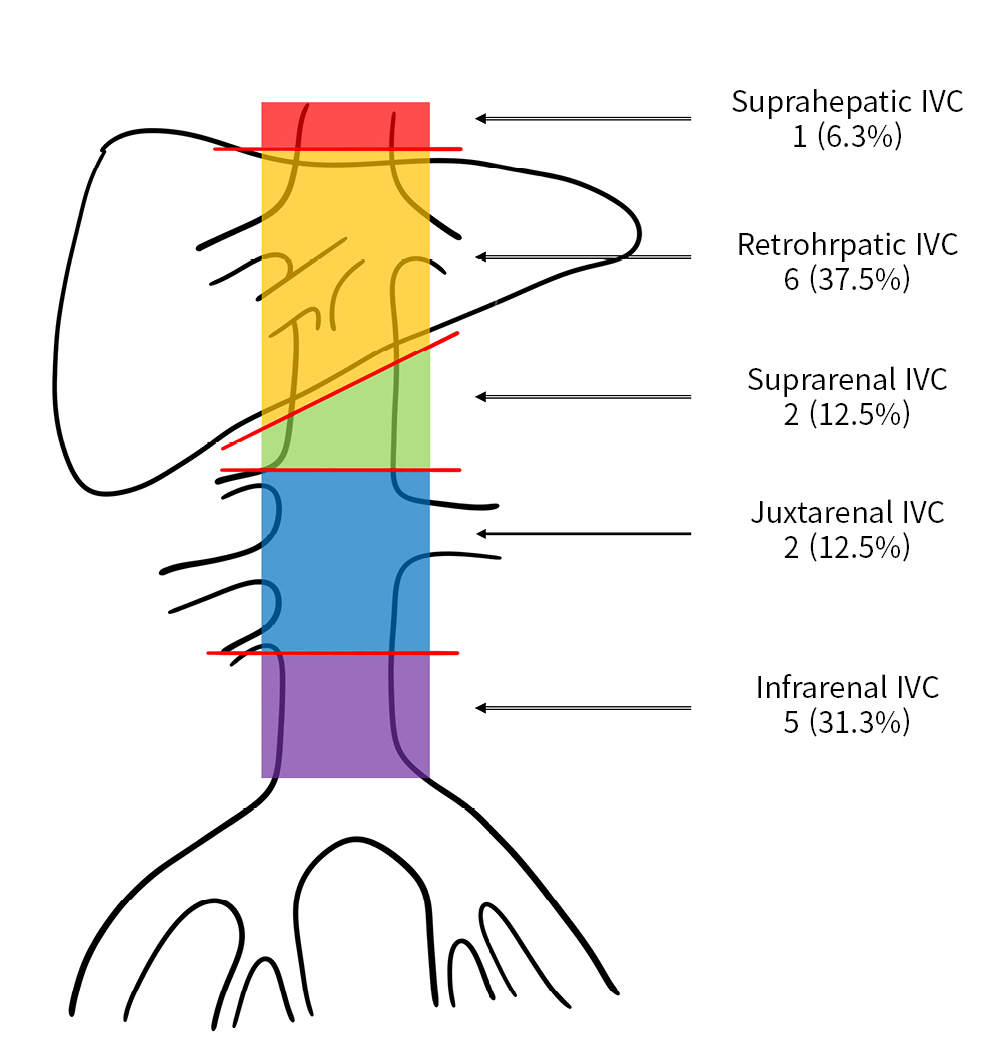
- The abdominal IVC was anatomically divided into five segments as follows: (1) suprahepatic IVC, from the upper margin of the hepatic veins to the lower margin of the diaphragm; (2) retrohepatic IVC, which is covered by the liver; (3) suprarenal IVC, from the upper margin of the renal vessels to the lower margin of the liver; (4) juxtarenal IVC, between the bilateral renal vessels; and (5) infrarenal IVC, from the bifurcation of the common iliac veins to the lower margin of the renal vessels (Fig. 2). In this study, subhepatic IVC included the IVC segments below the retrohepatic IVC.
- Statistical analysis
- Categorial variables were analyzed using the Fisher exact test and continuous variables were analyzed using the Mann-Whitney U-test. Continuous variables were expressed as median values (interquartile range, IQR). Statistical significance was set at P<0.05. Statistical analysis was conducted using R ver. 4.2.0 (R Foundation for Statistical Computing).
METHODS
- Demographics
- Of the 16 patients who had traumatic IVC injury, 10 (62.5%) were male and 15 (93.8%) had blunt trauma. The most common cause of traumatic IVC injury was a car-driver traffic accident (six patients, 37.5%), followed by falls (three patients, 18.8%). The median patient age was 48 years (IQR, 40–68 years), BMI was 23.4 kg/m2 (IQR, 21.0–26.1 kg/m2), prehospital time was 92.5 minutes (IQR, 54.0–158.0 minutes), and ISS was 34 (IQR, 25–43) (Table 1). The most common injury level was the retrohepatic IVC (six patients, 37.5%), followed by the infrarenal IVC (five patients, 31.3%), suprarenal IVC (two patients, 12.5%), juxtarenal IVC (two patients, 12.5%), and suprahepatic IVC (one patient, 6.3%) (Fig. 2).
- Clinical characteristics and initial laboratory findings
- During admission, the median SBP and MAP values were 86.5 mmHg (IQR, 71.8–120.8 mmHg) and 60.5 mmHg (IQR, 54.7–85.3 mmHg), respectively. Patients had a moderate loss of consciousness (median GCS, 10; IQR, 6–15). The initial laboratory data showed acidosis (median pH, 7.31; IQR, 7.24–7.37) with hyperlactatemia (median lactate, 4.3 mmol/L; IQR, 2.2–7.5 mmol/L) (Table 1).
- Treatment and surgical outcomes
- Of the 16 patients studied, eight (50.0%) underwent direct repair, two (12.5%) were closely monitored (observation), one (6.3%) underwent ligation, and one (6.3%) underwent an atriocaval shunt. Furthermore, four patients (25.0%) underwent other damage control surgery, and three (18.8%) underwent CPR in the ER. The median DTO time was 72.5 minutes (IQR, 55.8–109.8 minutes) and the median number of RBC units transfused within the first 24 hours was 29.0 (IQR, 19.8–40.3). Of the two patients who were closely monitored, one had a pericaval hematoma at the infrarenal IVC level and received only an injection of tranexamic acid and fluid therapy because the hemodynamics were stable. The other patient was hypotensive on arrival and had extravasation at the retrohepatic IVC level. However, the hemodynamics became stable after an initial transfusion of 2 units of RBCs. The patient was then closely monitored during hospitalization and received additional transfusions (14 units of RBCs, 7 units of fresh-frozen plasma, and 30 units of platelet concentrate) and injections of tranexamic acid. Both patients that were closely monitored, eventually survived. Of the 14 patients who underwent surgery, 11 (78.6%) did not survive, nine of whom (64.3%) died within 24 hours of admission (Table 1).
- Survivors who underwent damage control surgery
- The five survivors who underwent surgical treatment for traumatic IVC injury are presented in Table 2. All patients were injured at the subhepatic IVC level and successfully underwent direct repairs (venorrhaphy) in the first operation. Three patients were discharged alive and the remaining two died. One patient died of severe brain injury on hospital day 11, and the other died of septic shock with pneumonia on hospital day 12.
- Variables associated with surgical outcomes
- A BMI >23.0 kg/m2 (P=0.046), a higher serum lactate level (P=0.043), and a shorter operation time (P=0.016) were significantly associated with overall mortality (Table 3). A higher BMI (P=0.050), higher serum lactate level (P=0.004), shorter operation time (P=0.005), and injury at the retrohepatic IVC or higher level (P=0.031) were significantly associated with 24-hour mortality in the univariate analysis (Table 3).
- Comparison according to the injury level of the IVC
- Younger age (P=0.028), higher BMI (P=0.005), more acidic pH (P=0.028) with higher lactatemia (P=0.012), higher hemoglobin level (P=0.012), and shorter DTO time (P=0.028) were significantly associated with injury at the retrohepatic IVC or higher level (Table 4). Although not statistically significant, the MAP was lower (P=0.081) in patients with an injury at the retrohepatic IVC or higher level, and CPR in the ER was performed more frequently in these patients (P=0.055). All patients with injuries at the retrohepatic IVC or higher level who underwent surgical treatment died within 24 hours of admission. In contrast, patients with subhepatic IVC injuries had a high rate of direct repair (75.0%) and a significantly low 24-hour mortality rate (37.5%, P=0.031) (Table 4).
RESULTS
- In this study, the overall and 24-hour mortality rates after surgery were 78.6% and 64.3%, respectively. These outcomes are slightly worse than those noted in previous studies, where the overall mortality rates ranged between 20% and 70% [5,8,10–14]. The worse outcomes in this study are probably because 13 of the 14 patients who underwent surgery had blunt trauma, which is a known risk factor for mortality in traumatic IVC injury [7]. Of the nine deaths within 24 hours of admission seven developed cardiac arrest due to massive bleeding during the operation and did not recover. For the remaining two deceased patients, the operations ended with uncontrolled bleeding. Therefore, 24-hour mortality in this study indicated a failure of damage control.
- Several prognostic factors associated with traumatic IVC injury have been reported. According to previous studies, a higher IVC injury level was a prognostic factor for mortality [3,8]. A recent meta-analysis revealed that blunt trauma and injury at the suprarenal or higher IVC level were prognostic factors for mortality in traumatic IVC injury [7]. It is difficult to expose the retrohepatic IVC segment as the liver is attached to the anterior surface of the IVC, and the major hepatic veins connect to this level; therefore, the mortality rate associated with a retrohepatic IVC injury can be as high as 90% [3]. Furthermore, in this study, compared to patients with injury at the subhepatic IVC, those with injury at the retrohepatic IVC or higher level showed worse 24-hour mortality rates in the univariate analysis. In a study of 16 cases, multiple logistic regression analysis revealed lower GCS scores to be an independent factor for mortality [6]. We found that higher BMI and serum lactate levels were associated with mortality. Obesity has been reported as an independent risk factor for mortality in blunt trauma patients because the kinetic energy applied to the patient is in proportion to the mass; thus, obesity may increase the energy and cause more severe injury [15]. A higher initial serum lactate level has been associated with higher injury severity and can predict massive hemorrhage in trauma patients [16]. However, because the currently available studies and this study have all had small sample sizes, further studies with a large population are required to confirm that these are prognostic factors for traumatic IVC injury.
- The surgical treatments for IVC injuries vary depending on the location of the injury. In general, the first step is to expose the injured IVC segment and apply direct compression to the proximal and distal parts of the segment [1]. Direct repair can then be attempted following proximal and distal control of the injured IVC. However, when repair is difficult due to massive bleeding, ligation can be performed more quickly and easily for damage control [2]. In a study using data from the United States (2007 to 2014), excluding other-vessel injuries and severe extraperitoneal injuries, there was no difference in mortality between ligation and repair, and ligation was not an independent factor for mortality [17]. A recent meta-analysis showed that, compared with repair, ligation was associated with higher mortality. However, there was no significant difference in mortality rates for infrarenal IVC injuries [7]. Therefore, ligation appears to be a safe treatment option, particularly in infrarenal injuries. Nevertheless, we were unable to save a patient with an infrarenal IVC injury despite using ligation. For a retrohepatic IVC injury, liver mobilization may be required to expose the segment, but massive hemorrhage from the injury site can occur with this procedure [5]. Therefore, in a retrohepatic IVC injury, without active bleeding or with only a contained hematoma, perihepatic gauze packing without mobilization should be performed [1]. However, if packing fails to control the bleeding, direct repair of the injured site may be the only way to do so. Total hepatic vascular occlusion or an atriocaval shunt can be used to achieve a clear operative field during repair. Since subhepatic and suprahepatic IVC clamping for total hepatic vascular occlusion also blocks venous return, it may cause cardiac arrest. Therefore, additional aortic clamping at the supraceliac level or a venovenous bypass may be required, although outcomes have been reported to be poor [18,19]. Alternatively, an atriocaval shunt facilitates volume resuscitation during repair by isolating the injury site and maintaining venous return through a shunt using an endotracheal tube or a chest tube. As such, it can be used in a retrohepatic IVC injury with active bleeding, a large injury site, and extreme hemodynamic instability [4,5,20]. However, the reported outcomes of using an atriocaval shunt have also been unsatisfactory due to the severity of retrohepatic IVC injuries, the complexity of the procedure, the approach to the thorax, and delays in the decision to apply the procedure [4,20]. Richardson [21] reported a mortality rate of 88% in 412 patients with atriocaval shunts. We had one case of retrohepatic IVC injury for whom total hepatic vascular occlusion and an atriocaval shunt were ineffective. The patient was a 24-year-old man who was crushed by 2,000 kg of building material. Although venorrhaphy for a retrohepatic IVC injury was performed with total hepatic vascular occlusion, bleeding through an extended supradiaphragmatic IVC injury continued. By switching to an atriocaval shunt, the extended IVC injury was confirmed and venorrhaphy was successfully performed. Unfortunately, the patient did not survive because the delay in deciding to apply the atriocaval shunt resulted in significant blood loss (57 units of RBC were transfused during the operation). However, this case confirmed that an atriocaval shunt could be a better damage control tool than total hepatic vascular occlusion for primary repair of a retrohepatic IVC or higher level injury in terms of securing the operative field during massive bleeding.
- There were some limitations to this study. First, it was a retrospective study; therefore, important variables affecting surgical outcomes may have been missed. Moreover, data extracted from a review of medical records are not very reliable. Second, this was a single-center study, making it difficult to generalize the results to the overall population of patients with traumatic IVC injuries. Third, the statistical power of this study was weak because the sample size was small (14 to 16 patients).
- Subhepatic IVC injuries, which are easy to access, can be usually treated with a direct repair method. A systematic and multidisciplinary treatment strategy is required to deal with injuries at the retrohepatic IVC or higher level that are difficult to treat surgically. Since IVC injuries that require surgical treatment are rare, simulation training may be necessary to master the surgical skills needed for the complex damage control techniques (e.g., atriocaval shunt or ligation) and to understand their correct indications. This study described 14 cases in which surgical treatment was performed for IVC injury over a period of 9 years at a single institution. For a more comprehensive analysis, further studies are needed with larger populations and the participation of multiple centers.
DISCUSSION
-
Conflicts of interest
The authors have no conflicts of interest to declare.
-
Funding
None.
-
Data sharing statement
The data of this article are available from the corresponding author upon reasonable request.
-
Author contributions
Conceptualization: all authors; Data curation: all authors; Formal analysis: all authors; Investigation: all authors; Methodology: all authors; Project administration: DHK; Visualization: all authors; Writing–original draft: all authors; Writing–review & editing: DHK. All authors read and approved the final manuscript.
ARTICLE INFORMATION
| Variable | Value (n=16) |
|---|---|
| Male sex | 10 (62.5) |
| Age (yr) | 48 (40–68) |
| Body mass index (kg/m2) (n=14)a) | 23.4 (21.0–26.1) |
| Prehospital time (min) | 92.5 (54.0–158.0) |
| Injury mechanism (blunt) | 15 (93.8) |
| Cause of injury | |
| In-car TA | 6 (37.5) |
| Motorcycle TA | 2 (12.5) |
| Pedestrian TA | 2 (12.5) |
| Fall | 3 (18.8) |
| Crash injury | 2 (12.5) |
| Stab wound | 1 (6.3) |
| Injury Severity Score | 34 (25–43) |
| Abbreviated Injury Scale | |
| Head and neck | 0 (0–0) |
| Chest | 3 (2–3) |
| Abdominopelvic | 4 (4–5) |
| Extremities and pelvis | 0 (0–3) |
| Systolic blood pressure (mmHg) | 86.5 (71.8–120.8) |
| Mean arterial pressure (mmHg) | 60.5 (54.7–85.3) |
| Heart rate (beats/min) | 96.0 (76.5–115.0) |
| Glasgow Coma Scale | 10 (6–15) |
| pH | 7.31 (7.24–7.37) |
| PaO2 (mmHg) | 119.5 (60.8–209.3) |
| Lactate (mmol/L) | 4.3 (2.2–7.5) |
| Hemoglobin (g/dL) | 11.8 (9.0–13.1) |
| International normalized ratio (n=15)b) | 1.22 (1.08–1.37) |
| CPR in the ER | 3 (18.8) |
| DTO time (min) | 72.5 (55.8–109.8) |
| Treatment method | |
| Direct repair | 8 (50.0) |
| Ligation | 1 (6.3) |
| Atriocaval shunt | 1 (6.3) |
| Othersc) | 4 (25.0) |
| Observationd) | 2 (12.5) |
| Operation time (min) | 100.0 (80.5–157.5) |
| Transfused RBC within 24 hr (unit) | 29.0 (19.8–40.3) |
| Surgical outcome (n=14) | |
| Overall mortality | 11 (78.6) |
| 24-hr Mortality | 9 (64.3) |
Values are presented as median (interquartile range) or number (%).
TA, traffic accident; PaO2, partial pressure of arterial oxygen; CPR, cardiopulmonary resuscitation; ER, emergency room; DTO, door-to-operating room; RBC, red blood cell.
a) Values of two patients were not available.
b) Value of one patient was not available.
c) Gauze packing, manual compression, and resuscitative endovascular balloon occlusion of the aorta.
d) Medical treatment with close monitoring.
| Variable |
Overall mortality |
P-value |
24-hr Mortality |
P-value | ||
|---|---|---|---|---|---|---|
| Survival (n=3) | Death (n=11) | Survival (n=5) | Death (n=9) | |||
| Male sex | 1 (33.3) | 7 (63.6) | 0.539 | 2 (40.0) | 6 (66.7) | 0.580 |
| Age (yr) | 41.0 (40.5–57.5) | 48.0 (41.0–68.5) | 0.815 | 57 (41–68) | 47 (40–69) | 0.841 |
| Body mass index (kg/m2) | 20.8 (20.0–21.4) | 26.1 (23.3–26.1) | 0.094 | 21.5 (20.8–22.0) | 26.1 (25.4–27.7) | 0.050 |
| ≥23.0 | 0 | 7 (63.6) | 0.046 | 1 (20.0) | 6 (66.7) | 0.072 |
| Prehospital time (min) | 51.0 (37.0–121.0) | 93.0 (55.0–146.0) | 0.436 | 55.0 (51.0–70.0) | 130.0 (55.0–147.0) | 0.317 |
| Injury mechanism (blunt) | 2 (66.7) | 11 (100) | 0.214 | 4 (80.0) | 9 (100) | 0.357 |
| Injury Severity Score | 30 (28–36) | 34 (25–43) | 0.635 | 30 (25–42) | 34 (25–43) | 0.735 |
| Abbreviated Injury Scale | ||||||
| Head and neck | 0 (0–0) | 0 (0–0) | 0.523 | 0 (0–0) | 0 (0–0) | 0.662 |
| Chest | 3 (3–4) | 3 (2–3) | 0.665 | 3 (3–3) | 3 (0–3) | 0.504 |
| Abdominopelvic | 5 (5–5) | 4 (4–5) | 0.352 | 4 (4–5) | 4 (4–5) | 0.885 |
| Extremities and pelvis | 0 (0–0) | 0 (0–3) | 0.197 | 0 (0–0) | 2 (0–3) | 0.058 |
| Systolic blood pressure (mmHg) | 73.0 (66.0–104.0) | 82.0 (71.0–112.5) | >0.999 | 107.0 (73.0–131.0) | 78.0 (68.0–94.0) | 0.350 |
| Mean arterial pressure (mmHg) | 59.7 (52.3–79.3) | 61.3 (54.0–75.5) | >0.999 | 67.7 (59.7–99.0) | 56.0 (52.7–64.0) | 0.286 |
| Heart rate (beats/min) | 82.0 (82.0–95.5) | 102.0 (71.5–131.0) | 0.755 | 82.0 (82.0–109.0) | 102.0 (72.0–130.0) | 0.894 |
| Glasgow Coma Scale | 15 (11–15) | 9 (6–13) | 0.423 | 6 (6–15) | 10 (7–15) | 0.891 |
| pH | 7.37 (7.34–7.39) | 7.27 (7.14–7.34) | 0.119 | 7.37 (7.31–7.40) | 7.27 (7.12–7.31) | 0.061 |
| PaO2 (mmHg) | 194.0 (125.0–239.5) | 80.0 (60.5–209.5) | 0.815 | 56.0 (56.0–194.0) | 82.0 (76.0–210.0) | 0.229 |
| Lactate (mmol/L) | 2.3 (2.2–2.4) | 5.2 (4.3–9.9) | 0.043 | 2.3 (2.0–2.4) | 7.3 (4.9–11.6) | 0.004 |
| Hemoglobin (g/dL) | 10.5 (9.5–10.7) | 11.9 (8.9–13.1) | 0.350 | 10.5 (8.5–10.8) | 13.0 (9.0–13.1) | 0.182 |
| International normalized ratio | 1.05 (0.99–1.14) | 1.24 (1.13–1.43) | 0.127 | 1.19 (1.05–1.22) | 1.25 (1.11–1.51) | 0.163 |
| CPR in ER | 0 | 3 (27.3) | >0.999 | 0 | 3 (33.3) | 0.258 |
| DTO time (min) | 69.0 (68.0–99.0) | 76.0 (51.0–101.5) | 0.696 | 76.0 (69.0–129.0) | 67.0 (50.0–85.0) | 0.181 |
| Treatment method | 0.692 | 0.119 | ||||
| Direct repair | 3 (100) | 5 (45.5) | 5 (100) | 3 (33.3) | ||
| Ligation | 0 | 1 (9.1) | 0 | 1 (11.1) | ||
| Atriocaval shunt | 0 | 1 (9.1) | 0 | 1 (11.1) | ||
| Othersa) | 0 | 4 (36.4) | 0 | 4 (44.4) | ||
| Operation time (min) | 200.0 (180.0–232.0) | 90.0 (80.0–111.0) | 0.016 | 160.0 (160.0–200.0) | 82.0 (80.0–95.0) | 0.005 |
| Transfused RBC within 24 hr (unit) | 40.0 (35.0–40.5) | 28.0 (1.5–40.5) | 0.456 | 36.0 (30.0–40.0) | 28.0 (22.0–45.0) | 0.797 |
| IVC injury level | 0.368 | 0.091 | ||||
| Suprahepatic | 0 | 1 (9.1) | 0 | 1 (11.1) | ||
| Retrohepatic | 0 | 5 (45.5) | 0 | 5 (55.6) | ||
| Suprarenal | 1 (33.3) | 1 (9.1) | 2 (40.0) | 0 | ||
| Juxtarenal | 1 (33.3) | 1 (9.1) | 1 (20.0) | 1 (11.1) | ||
| Infrarenal | 1 (33.3) | 3 (27.3) | 2 (40.0) | 2 (22.2) | ||
| Level (retrohepatic or higher) | 0 | 6 (54.5) | 0.209 | 0 | 6 (66.7) | 0.031 |
Values are presented as number (%) or median (interquartile range).
PaO2, partial pressure of arterial oxygen; CPR, cardiopulmonary resuscitation; ER, emergency room; DTO, door-to-operating room; RBC, red blood cell; IVC, inferior vena cava.
a) Gauze packing, manual compression, and resuscitative endovascular balloon occlusion of the aorta.
| Variable | Retrohepatic IVC or higher (n = 6) | Subhepatic IVC (n = 8) | P-value |
|---|---|---|---|
| Surgical outcome | |||
| Overall mortality | 6 (100) | 5 (62.5) | 0.209 |
| 24-hr Mortality | 6 (100) | 3 (37.5) | 0.031 |
| Male sex | 5 (83.3) | 3 (37.5) | 0.138 |
| Age (yr) | 41 (33–46) | 69 (53–75) | 0.028 |
| Body mass index (kg/m2) | 26.1 (26.1–28.5) | 21.1 (19.6–21.9) | 0.005 |
| Prehospital time (min) | 74.0 (47.5–120.8) | 108.5 (54.0–198.3) | 0.366 |
| Injury mechanism (blunt) | 6 (100) | 7 (87.5) | >0.999 |
| Injury Severity Score | 43 (36–43) | 28 (25–36) | 0.101 |
| Abbreviated Injury Scale | |||
| Head and neck | 0 (0–0) | 0 (0–1) | 0.244 |
| Chest | 3 (3–3) | 3 (2–3) | 0.518 |
| Abdominopelvic | 5 (4–5) | 4 (4–4) | 0.326 |
| Extremities and pelvis | 2 (0–3) | 0 (0–1) | 0.285 |
| Systolic blood pressure (mmHg) | 76.0 (18.5–81.0) | 100.5 (71.8–121.3) | 0.272 |
| Mean arterial pressure (mmHg) | 54.0 (13.2–59.8) | 65.8 (58.8–99.9) | 0.081 |
| Heart rate (beats/min) | 101.0 (60.3–108.0) | 95.5 (79.5–130.5) | 0.747 |
| Glasgow Coma Scale | 9 (4–14) | 9 (6–15) | 0.740 |
| pH | 7.14 (7.08–7.24) | 7.34 (7.31–7.38) | 0.028 |
| PaO2 (mmHg) | 81.0 (77.0–178.0) | 127.0 (56.0–228.0) | 0.651 |
| Lactate (mmol/L) | 9.9 (5.7–13.1) | 3.3 (2.2–4.5) | 0.011 |
| Hemoglobin (g/dL) | 13.1 (13.0–14.1) | 9.7 (8.0–11.0) | 0.011 |
| International normalized ratio | 1.25 (1.10–1.25) | 1.21 (1.10–1.30) | 0.942 |
| CPR in ER | 3 (50.0) | 0 | 0.055 |
| DTO time (min) | 51.0 (47.8–63.3) | 80.5 (74.3–120.8) | 0.028 |
| Treatment method | 0.277 | ||
| Direct repair | 2 (33.3) | 6 (75.0) | |
| Ligation | 0 | 1 (12.5) | |
| Atriocaval shunt | 1 (16.7) | 0 | |
| Othersa) | 3 (50.0) | 1 (12.5) | |
| Operation time (min) | 92.5 (82.5–102.5) | 138.5 (81.5–170.0) | 0.244 |
| Transfused RBC within 24 hr (unit) | 36.0 (18.8–48.8) | 32.5 (26.5–37.0) | 0.852 |
Values are presented as median (interquartile range) or number (%).
IVC, inferior vena cava; PaO2, partial pressure of arterial oxygen; CPR, cardiopulmonary resuscitation; ER, emergency room; DTO, door-to-operating room; RBC, red blood cell.
a) Gauze packing, manual compression, and resuscitative endovascular balloon occlusion of the aorta.
- 1. Rehman ZU. Inferior vena cava injuries: a clinical review. J Pak Med Assoc 2020;70:1069–71. PubMed
- 2. Matsumoto S, Jung K, Smith A, Coimbra R. Management of IVC injury: repair or ligation? A propensity score matching analysis using the National Trauma Data Bank. J Am Coll Surg 2018;226:752–9. ArticlePubMed
- 3. Sullivan PS, Dente CJ, Patel S, et al. Outcome of ligation of the inferior vena cava in the modern era. Am J Surg 2010;199:500–6. ArticlePubMed
- 4. Burch JM, Feliciano DV, Mattox KL. The atriocaval shunt: facts and fiction. Ann Surg 1988;207:555–68. ArticlePubMedPMC
- 5. Castater CA, Carlin M, Parker VD, et al. Intra-abdominal inferior vena cava injuries: operative strategies and outcomes. Am Surg 2021;87:1316–26. ArticlePubMedPDF
- 6. Cudworth M, Fulle A, Ramos JP, Arriagada I. GCS as a predictor of mortality in patients with traumatic inferior vena cava injuries: a retrospective review of 16 cases. World J Emerg Surg 2013;8:59. ArticlePubMedPMCPDF
- 7. Byerly S, Tamariz L, Lee EE, et al. A systematic review and meta-analysis of ligation versus repair of inferior vena cava injuries. Ann Vasc Surg 2021;75:489–96. ArticlePubMed
- 8. Rosengart MR, Smith DR, Melton SM, May AK, Rue LW 3rd. Prognostic factors in patients with inferior vena cava injuries. Am Surg 1999;65:849–56. ArticlePubMedPDF
- 9. Meredith W, Rutledge R, Fakhry SM, Emery S, Kromhout-Schiro S. The conundrum of the Glasgow Coma Scale in intubated patients: a linear regression prediction of the Glasgow verbal score from the Glasgow eye and motor scores. J Trauma 1998;44:839–45. PubMed
- 10. Graham JM, Mattox KL, Beall AC Jr, DeBakey ME. Traumatic injuries of the inferior vena cava. Arch Surg 1978;113:413–8. ArticlePubMed
- 11. Navsaria PH, de Bruyn P, Nicol AJ. Penetrating abdominal vena cava injuries. Eur J Vasc Endovasc Surg 2005;30:499–503. ArticlePubMed
- 12. Tsai R, Raptis C, Schuerer DJ, Mellnick VM. CT appearance of traumatic inferior vena cava injury. AJR Am J Roentgenol 2016;207:705–11. ArticlePubMed
- 13. Giannakopoulos TG, Avgerinos ED. Management of peripheral and truncal venous injuries. Front Surg 2017;4:46. ArticlePubMedPMC
- 14. van Rooyen PL, Karusseit VO, Mokoena T. Inferior vena cava injuries: a case series and review of the South African experience. Injury 2015;46:71–5. ArticlePubMed
- 15. Brahmbhatt TS, Hernon M, Siegert CJ, Plauche L, Young LS, Burke P. Trauma and BMI mortality. Curr Obes Rep 2017;6:211–6. ArticlePubMedPDF
- 16. Baxter J, Cranfield KR, Clark G, Harris T, Bloom B, Gray AJ. Do lactate levels in the emergency department predict outcome in adult trauma patients? A systematic review. J Trauma Acute Care Surg 2016;81:555–66. ArticlePubMed
- 17. Byerly S, Cheng V, Plotkin A, Matsushima K, Inaba K, Magee GA. Impact of inferior vena cava ligation on mortality in trauma patients. J Vasc Surg Venous Lymphat Disord 2019;7:793–800. ArticlePubMed
- 18. Kobayashi LM, Costantini TW, Hamel MG, Dierksheide JE, Coimbra R. Abdominal vascular trauma. Trauma Surg Acute Care Open 2016;1:e000015ArticlePubMedPMC
- 19. Chaib E, Saad WA, Fujimura I, Saad WA Jr, Gama-Rodrigues J. The main indications and techniques for vascular exclusion of the liver. Arq Gastroenterol 2003;40:131–6. ArticlePubMed
- 20. Richardson JD, Franklin GA, Lukan JK, et al. Evolution in the management of hepatic trauma: a 25-year perspective. Ann Surg 2000;232:324–30. ArticlePubMedPMC
- 21. Richardson JD. Changes in the management of injuries to the liver and spleen. J Am Coll Surg 2005;200:648–69. ArticlePubMed
REFERENCES
Figure & Data
References
Citations

 KST
KST

 PubReader
PubReader ePub Link
ePub Link Cite
Cite