Effects of Massive Transfusion Protocol Implementation in Trauma Patients at a Level I Trauma Center
Article information
Abstract
Purpose
This study was conducted to investigate whether rapid and efficient administration of blood products was achieved and whether clinical outcomes were improved by applying a massive transfusion protocol (MTP).
Methods
From January 2016 to September 2019, the medical records of trauma patients who received at least 10 units of packed red blood cells (PRBC) at Pusan National University Hospital (level I trauma center) were retrospectively reviewed. The patients treated from January 2016 to January 2018 were designated as the non-MTP group, and those treated from February 2018 to September 2019 were designated as the MTP group.
Results
During the study period, 370 patients received massive transfusions. The non-MTP and MTP groups comprised 84 and 55 patients, respectively. No significant between-group differences were found in the units of PRBC (23.2 vs. 25.3, respectively; p=0.46), fresh frozen plasma (FFP) (21.1 vs. 24.4, respectively; p=0.40), and platelets (PLT) (15.4 vs. 17.0, respectively; p=0.54) administered in the first 24 hours. No statistically significant differences between the non-MTP and MTP groups were found in the FFP-to-PRBC ratio (0.9 vs. 0.94, respectively; p=0.44) and or the PLT-to-PRBC ratio (0.72 vs. 0.72, respectively; p=0.21). However, the total number of cryoprecipitate units was significantly higher in the MTP group than in the non-MTP group (7.4 vs. 15.3 units, respectively; p=0.003) and the ratio of cryoprecipitate to PRBC in the MTP group was significantly higher than in the non-MTP group (0.31 vs. 0.62, respectively; p=0.021). The time to transfusion was significantly reduced after MTP implementation (41.0 vs. 14.9 minutes, respectively; p=0.003).
Conclusions
Although no significant differences were found in the clinical outcomes of patients who had undergone severe trauma, rapid and balanced transfusion was achieved after implementing the MTP.
INTRODUCTION
Massive transfusion is commonly defined as a transfusion of more than 10 units of packed red blood cells (PRBC) in 24 hours [1-3]. Crystalloid solutions are commonly used during resuscitation of patients who have undergone trauma with hemorrhagic shock. However, the rapid administration of blood products is currently encouraged. In addition, balanced transfusions of PRBC, fresh frozen plasma (FFP), and platelets (PLT) are known to improve coagulopathy caused by hemorrhagic shock. Several studies have shown that administering PRBC, FFP, and PLT at a ratio of 1:1:1 or higher reduces mortality and improves treatment outcomes [4-6]. A massive transfusion protocol (MTP) is required for efficient massive transfusion, as it aims to rapidly and effectively deliver the blood products that are necessary for trauma resuscitation. Many trauma centers have implemented MTPs to deliver blood products, resulting in improved patient outcomes and mortality [7-9]. Accordingly, we also introduced a MTP in February 2018 and have applied it in the context of massive transfusions in severe trauma patients. The purpose of this study was to determine whether rapid and efficient administration of blood products was achieved by applying the MTP, and whether mortality and clinical outcomes were improved through this approach.
METHODS
The medical records of patients who received greater than 10 units of PRBC during the first 24 hours after presenting to the emergency department of the trauma center of Pusan National University Hospital from January 2016 to September 2019 were retrospectively analyzed. The institution’s MTP was implemented in February 2018. Patients were divided into a non-MTP group and an MTP group according to whether they were treated before or after implementation of the MTP. The MTP group comprised only patients transfused by applying MTP who received more than 10 units of PRBC. In both groups, patients who were transferred after initiation of blood transfusion at another institution, children (under 18 years of age), and patients without abdominal injuries were excluded. The reason for excluding patients without abdominal injuries is that abdominal injuries are the most common indication for massive transfusion and the most common cause of death from hypovolemic shock. In addition, excluding orthopedic and neurosurgery patients served as a way to control for potential inconsistencies in the process of patient treatment.
The demographic and clinical data collected included age, sex, injury severity score (ISS), abdominal organ injury scale (OIS), mechanism of injury (blunt or penetrating), Glasgow Coma Scale (GCS), duration to death, cause of death, total blood product count, time to transfusion, intensive care unit (ICU) length of stay (LOS), hospital LOS, and number of days on mechanical ventilation. At Pusan National University Hospital, the MTP consists of three stages of delivery, and MTP activation is indicated in cases where active bleeding is found or where there is either no response or a minimal response of vital signs despite initial fluid resuscitation (Fig. 1). When a patient arrives at the trauma bay and meets the MTP activation criteria, the MTP is activated by the emergency medical staff and tranexamic acid is simultaneously administered. Boxes 1 and 2 supply O+ blood products without a screening test before transfusion, allowing rapid transfusion. Usually, by the time that box 3 is administered, the screening test has been completed and blood products suitable for the patient are supplied. After box 2, laboratory tests (complete blood count, prothrombin time, activated partial thromboplastic time, fibrinogen, ionized calcium levels, electrolyte levels, arterial blood gas analysis, and lactic acid levels) are performed, and box 3 is repeatedly administered until sufficient resuscitation is achieved.
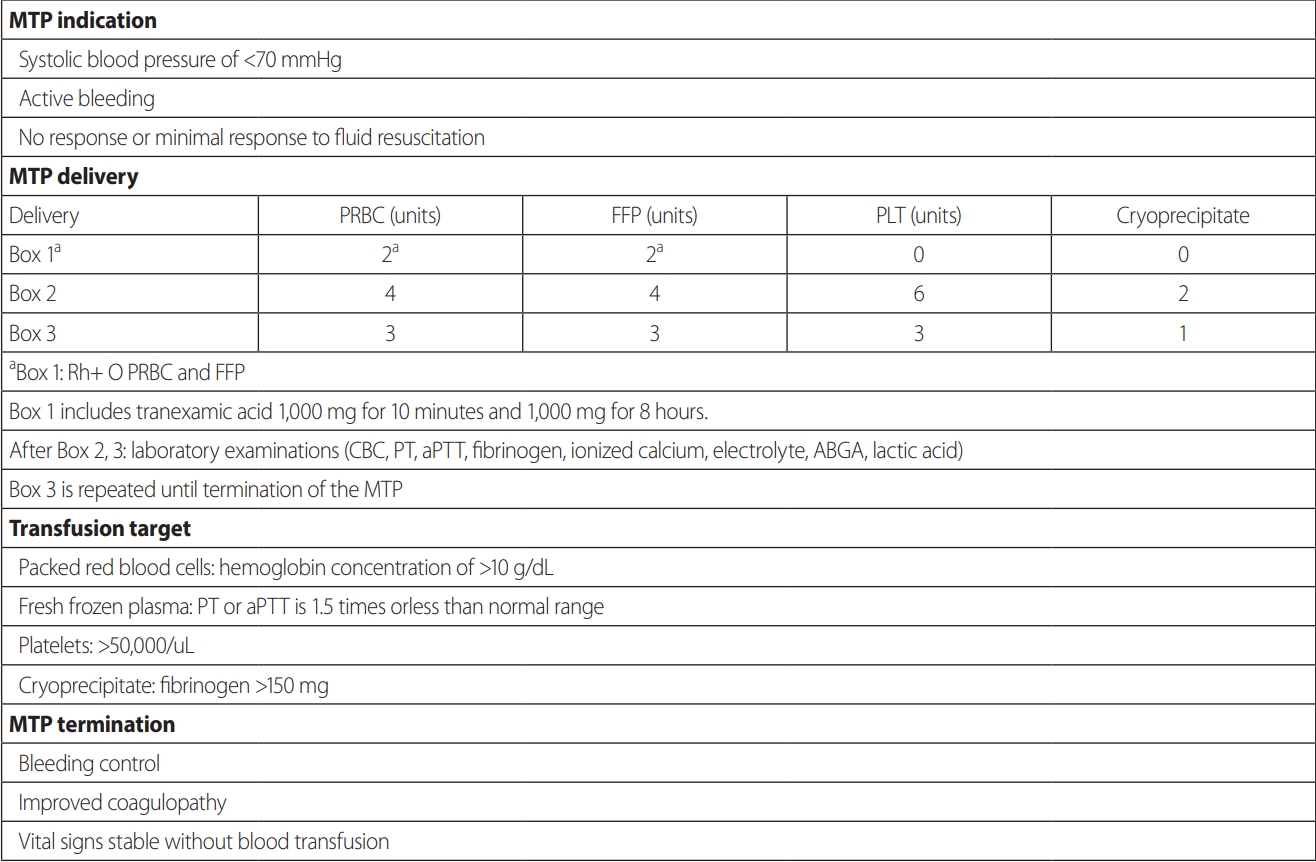
Massive transfusion protocol at Pusan National University Hospital, a level I trauma center. MTP: massive transfusion protocol, PRBC: packed red blood cells, FFP: fresh frozen plasma, PLT: platelets, CBC: complete blood count, PT: prothrombin time, aPTT: activated partial thromboplastin time, ABGA: arterial blood gas analysis.
The total count of PRBC, FFP, PLT, and cryoprecipitate units administered during the 24-hour period from the time of arrival at the emergency department was recorded for each patient, and the ratios of FFP to PRBC, PLT to PRBC, and cryoprecipitate to PRBC were calculated. The count of total blood products and differences in the ratios of FFP to PRBC, PLT to PRBC, and cryoprecipitate to PRBC administered to the non-MTP and MTP groups were compared. We also compared the mortality rate, mortality due to hypovolemic shock, time to transfusion, ICU LOS, hospital LOS, and the duration of mechanical ventilation between the two groups. This study was approved by the Institutional Review Board of Pusan National University Hospital (IRB No. H-1910-038-084).
Statistical analyses were performed using SPSS version 21.0 (IBM Corp., Armonk, NY, USA). The Student t-test was used to compare the means of continuous variables. The chi-square test was used to analyze categorical data, and the z-test was used to compare the two populations. A p-value of <0.05 was considered to indicate statistical significance.
RESULTS
From January 2016 to September 2019, a total of 370 patients received massive transfusions at the trauma center of Pusan National University Hospital. Of the 194 patients who received treatment before the MTP was implemented, 84 patients were analyzed as part of the non-MTP group. Six patients under the age of 18 years, 21 patients who started transfusion at other institutions, and 83 patients without abdominal injuries were excluded. Of the 176 patients treated after the MTP was implemented, 55 patients were analyzed as part of the MTP group. Fifteen patients under the age of 18 years, 13 patients who started transfusion at other institutions, 81 patients who underwent massive transfusion without MTP activation, and 12 patients without abdominal injuries were excluded.
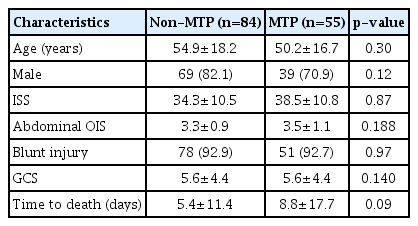
The demographic and clinical characteristics of the two groups are shown in Table 1. Age, sex, ISS, abdominal OIS, mechanism of injury, and GCS were not significantly different between the two groups. Although the time to death was slightly longer in the MTP group, there was no statistically significant difference (5.4 days vs. 8.8 days, respectively; p=0.09).
The total count of PRBC (23.2 units vs. 25.3 units, respectively; p=0.46), FFP (21.1 units vs. 24.4 units, respectively; p=0.40), and PLT (15.4 units vs. 17.0 units, respectively; p=0.54) administered in the first 24 hours was not significantly different between the two groups. However, the total count of cryoprecipitate was significantly higher in the MTP group (7.4 units vs. 15.3 units, respectively; p=0.003).
The ratio of FFP to PRBC (0.9 vs. 0.94, respectively; p=0.44) and the ratio of PLT to PRBC (0.72 vs. 0.72, respectively; p=0.21) did not show statistically significant differences before and after MTP implementation. However, the ratio of cryoprecipitate to PRBC in the MTP group increased significantly (0.31 vs. 0.62, respectively; p=0.021) (Table 2).

Amount and ratio of blood products administered to the non-MTP and MTP groups for the first 24 hours.
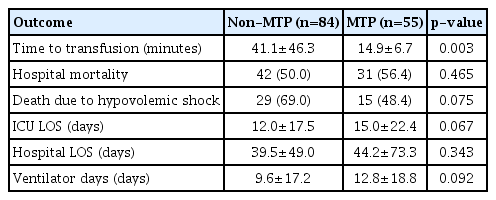
The time to transfusion was significantly lower in the MTP group (41.0 minutes vs. 14.9 minutes, respectively; p=0.003). Hospital mortality (50.0% vs. 56.4%, respectively; p=0.465) and hospital LOS (39.5 days vs. 44.2 days, respectively; p=0.343) were not significantly different between the two groups.
The period of mechanical ventilation (9.6 days vs. 12.8 days, respectively; p=0.092) and ICU LOS (12.0 days vs. 15.0 days, respectively; p=0.067) were slightly longer in the MTP group than in the non-MTP group, but no statistically significant difference was observed. The mortality rate due to hypovolemic shock was also slightly lower in the MTP group than in the non-MTP group, but no statistically significant difference was observed (69.0% vs. 48.4%, respectively; p=0.075) (Table 3).
DISCUSSION
Hemorrhage is the leading cause of death in patients with severe trauma and has a high mortality rate (39–54%) [10,11]. Continuous hemorrhage causes a lethal triad of hypothermia, metabolic acidosis, and coagulopathy, and can lead to death if rapid correction of hypovolemia is not achieved [12]. Crystalloids, such as lactated Ringer’s or normal saline, have been widely used in trauma resuscitation because they are easily accessible. However, crystalloids not only exacerbate the lethal triad, but also lead to the development of abdominal compartment syndrome, cardiac complications, and pulmonary complications. Therefore, the use of crystalloids should be reduced during resuscitation and blood products should be administered as soon as possible. This approach improves patients’ clinical outcomes [13,14]. In the last 20 years, consensus has been reached on the optimal rate of blood product administration during massive transfusion, and the implementation of an MTP for the rapid and efficient delivery of blood products is essential. Several studies have reported that the implementation of MTPs reduced mortality, hospital LOS, and ICU LOS [14-16].
To implement an MTP, it is necessary to make efforts in accordance with the medical environment of each institution, which requires sufficient support and agreement between the relevant departments (i.e., the department of emergency medicine, trauma surgeons, the department of laboratory medicine, and the institutional blood bank) [17]. Korea is in the early stages of introducing its trauma system. The establishment of trauma centers began in 2012, led by the government, and there were few wellestablished MTPs [18,19].
The trauma center of Pusan National University Hospital was established in 2015, and an MTP was implemented in February 2018 after consultation among related departments. Our MTP drew upon previous studies and specifies a 1:1:1 ratio of PRBC, FFP, and PLT [20,21]. After MTP box 2 is used, laboratory tests are performed to enable more accurate blood transfusion. Before MTP implementation, fibrinogen was administered according to laboratory findings, but it is known that proper administration of cryoprecipitate improves patients’ clinical outcomes [22,23]. Although cryoprecipitate is included in the MTP, the ratio of cryoprecipitate was low because consultations with other departments were not sufficient at the initial stages of MTP introduction. We are trying to improve the protocol to increase the volume of cryoprecipitate that is administered.
In this study, the total count of PRBC, FFP, and PLT units administered before and after MTP implementation was not significantly different. The FFP-to-PRBC ratio and the PLT-to-PRBC ratio did not show statistically significant changes after the implementation of the MTP. However, the total count of cryoprecipitate units and the cryoprecipitate-to-PRBC ratio increased significantly in the MTP group. The FFP-to-PRBC and PLTto-PRBC ratios did not change significantly because even before implementation of the MTP, the need for balanced transfusions was widely understood. However, since cryoprecipitate was administered according to the results of laboratory tests prior to implementation of the MTP, balanced transfusion was not achieved.
The most significant result after implementation of the MTP was that the average time to transfusion decreased from 41.1 minutes to 14.9 minutes (p=0.003). In the resuscitation of patients with traumatic hypovolemic shock, the rapid administration of blood products, rather than crystalloids, is the basic principle of trauma resuscitation. Therefore, this difference reflects a clinically important benefit for the patients in the MTP group. However, no differences were found between the two groups in terms of mortality and hospital LOS before and after implementation of the MTP. Furthermore, the ICU LOS and mechanical ventilation period were slightly longer in the MTP group than in the non-MTP group, although this difference was not significant. In addition, mortality due to hypovolemic shock decreased slightly (69.0% vs. 48.4%, respectively; p=0.075) and the time to death was slightly extended after implementation of the MTP (5.4 days vs. 8.4 days, respectively; p=0.09). It is thought that the time to transfusion shortened due to the rapid delivery of blood products after implementation of the MTP, and the treatment opportunities of severe trauma patients increased, resulting in a slight reduction in mortality due to hypovolemic shock and a prolonged time to death.
The present study has some limitations. First, the study adopted a retrospective design; therefore, it was not possible to accurately measure the total amount of crystalloids administered during the first 24 hours. This is a potentially important issue, since the use of crystalloids during resuscitation exacerbates the lethal triad, but it was not possible to determine how crystalloid use affected patients’ clinical outcomes.
Second, the duration of MTP implementation was short. Therefore, the period to demonstrate improvement in patients’ clinical outcomes was limited. Time will also be required for all medical staff involved in trauma resuscitation to adopt the new protocol. Even after the MTP was implemented, more patients underwent massive transfusion without MTP activation than underwent massive transfusion according to the MTP. Therefore, educating medical staff is of paramount importance, and efforts will be required to apply the protocol to all patients who are expected to undergo massive transfusion.
Finally, there may have been patients who died without the opportunity for treatment because the time to transfusion was delayed before implementation of the MTP. If further studies involving these patients are conducted, it will likely be found that the MTP is useful and improves clinical outcomes for severe trauma patients.
CONCLUSION
Although there was no significant difference in the clinical outcomes of patients who had undergone severe trauma, rapid and balanced transfusion was achieved after implementing the MTP. The protocol will need to be modified to ensure balanced transfusion, and further studies are needed to confirm the improvement in clinical outcomes in these patients.
Acknowledgements
This work was supported by clinical research grant form Pusan National University Hospital in 2020.