Articles
- Page Path
- HOME > J Trauma Inj > Volume 33(4); 2020 > Article
-
Original Article
A Reappraisal of the Necessity of a Ventriculoperitoneal Shunt After Decompressive Craniectomy in Traumatic Brain Injury - Seunghan Yu, M.D., Hyuk Jin Choi, M.D., Jung Hwan Lee, M.D., Mahnjeong Ha, M.D., Byung Chul Kim, M.D.
-
Journal of Trauma and Injury 2020;33(4):236-241.
DOI: https://doi.org/10.20408/jti.2020.0072
Published online: December 31, 2020
- 3,342 Views
- 111 Download
- 1 Crossref
Department of Neurosurgery, Medical Research Institute, Pusan National University Hospital, Busan, Korea
- Correspondence to Byung Chul Kim, M.D., Department of Neurosurgery, Pusan, National University Hospital, 179 Gudeokro, Seo-gu, Busan 49241, Korea Tel: +82-51-240-7257, Fax: +82-51-244-0282, E-mail: bc1743kim@naver.com
- * SY and HJC contributed equally as first authors.
• Received: November 3, 2020 • Revised: November 30, 2020 • Accepted: December 4, 2020
Copyright © 2020 The Korean Society of Trauma
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Purpose
- Cranioplasty itself is believed to have therapeutic effects on hydrocephalus. The goal of this study was to evaluate the hypothesis that not every patient with hydrocephalus after decompressive craniectomy needs cerebrospinal fluid diversion, and that cranioplasty should be performed before considering cerebrospinal fluid diversion.
-
Methods
- Data were collected from 67 individual traumatic brain injury patients who underwent cranioplasty between January 1, 2019 and December 31, 2019. Patients’ clinical and radiographic progression was reviewed retrospectively based on their medical records.
-
Results
- Twenty-two of the 67 patients (32.8%) had ventriculomegaly on computed tomography scans before cranioplasty. Furthermore, 38 patients showed progressive ventriculomegaly after cranioplasty. Of these 38 patients, only six (15.7%) showed worsening neurologic symptoms, which were improved by the tap test; these patients eventually underwent ventriculoperitoneal shunt placement.
-
Conclusions
- Cerebrospinal fluid diversion is not always required for radiologically diagnosed ventriculomegaly in traumatic brain injury patients after decompressive craniectomy. A careful clinical and neurologic evaluation should be conducted before placing a shunt.
- Decompressive craniectomy (DC) is frequently performed in patients with severely increased intracranial pressure due to several conditions, including traumatic brain injury (TBI) [1–6]. During the recovery period, cranioplasty (CP) is often indicated for protective and cosmetic purposes [2]. Furthermore, cranial defects that occur after DC can lead to syndrome of the trephined and additional injury [7,8].
- Meanwhile, many patients also show hydrocephalus after DC; post-traumatic hydrocephalus has been reported to occur in 0.7–86% of patients [9,10]. The mechanism of hydrocephalus after DC has not been clearly identified, but in addition to the initial insult, a large cranial defect could lead to turbulence in the hydrodynamics of cerebrospinal fluid (CSF) circulation and cerebral perfusion due to exposure to atmospheric pressure, followed by hydrocephalus [11–15]. If the aforementioned hypothesis is true, CP itself has therapeutic effects on hydrocephalus [12,13,16]. However, the current indications for CP and various CSF techniques for diversion, including ventriculoperitoneal shunt (VPS) placement, are largely subjective and based on personal or institutional preferences. In some cases, CP and VPS are performed simultaneously despite the well-known risks and complications of VPS. We hypothesized that not every patient with hydrocephalus after DC needs VPS, and that CP should be performed before considering VPS.
INTRODUCTION
- Data were collected from 71 individual TBI patients who underwent CP in a regional trauma center of Pusan National University Hospital between January 1, 2019, and December 31, 2019. Of the 71 patients, four patients who underwent CP were excluded because of postoperative infections. As a result, 67 patients were enrolled. Radiologic data and patients’ neurologic progression were reviewed retrospectively through their electronic medical records.
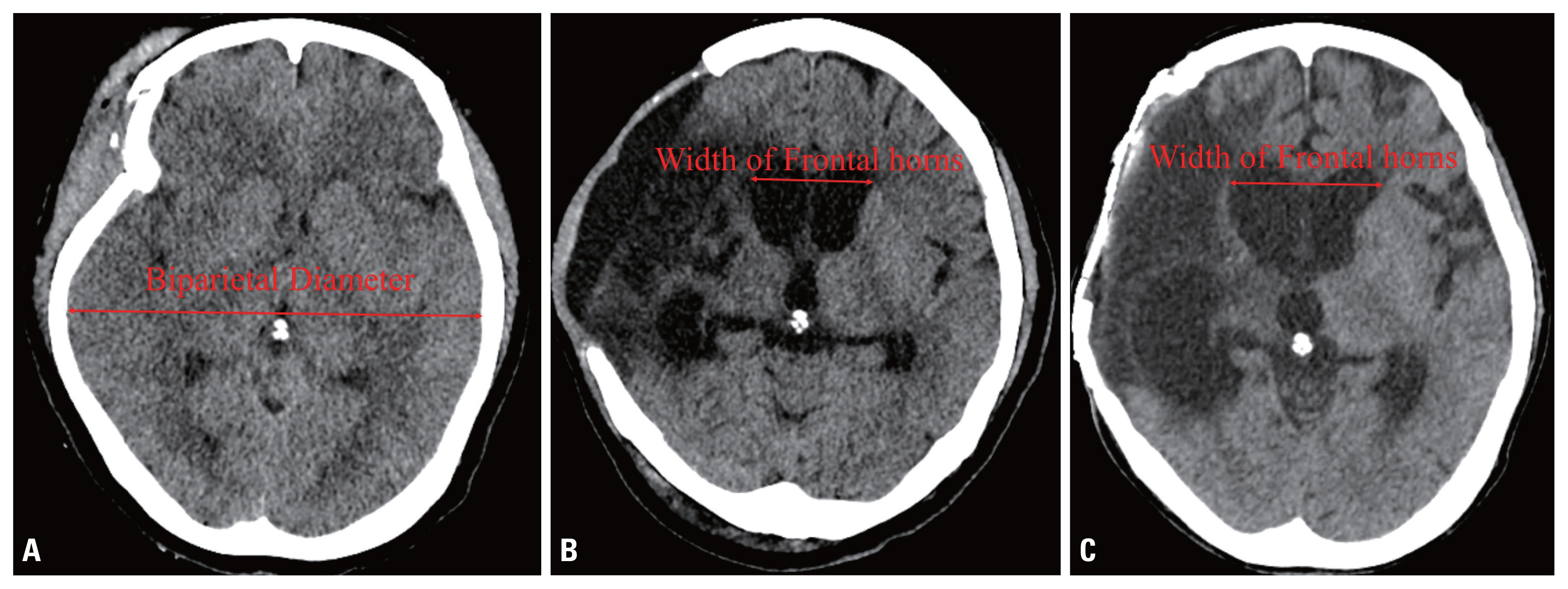
- To evaluate of the necessity of VPS placement, the size of the ventricle and the patient’s neurologic condition were checked. Hydrocephalus after TBI was defined as 1) an Evans index greater than 0.3 on brain computed tomography (CT); 2) a progressive increase in ventricular size; 3) periventricular white matter showing low density on CT; and 4) neurologic improvement after the withdrawal of CSF via a tap test (Fig. 1). The neurologic evaluation was performed using the Rancho Los Amigos scale.
- CP was performed on all patients using autologous bone stored in the bone bank. To compare ventriculomegaly before and after CP, the Evans index (the largest width of the frontal horns to the maximal biparietal diameter measured in the same CT slice) was checked just before CP and about 1 month after CP. Because of skull bone defects after DC, the maximal biparietal diameter was measured on the initial CT scan at the time of admission. A tap test was performed in patients with worsening neurologic symptoms or advanced hydrocephalus on follow-up CT. Approximately 50 cc of CSF was removed from the patient through a lumbar puncture. If the tap test led to symptom improvement, it could be predicted that VPS placement would have positive effects. Therefore, when a patient showed neurologic improvement after the tap test, VPS placement was performed. Otherwise, the patient was monitored at the outpatient department on a regular basis.
- Correlations between various radiographic parameters regarding the degree of ventriculomegaly and VPS were examined using the Mann-Whitney test. The Fisher exact test was employed to analyze correlations between the prevalence of preoperative ventriculomegaly and VPS. All statistical analyses were conducted with the SPSS statistical software package version 22.0 (IBM Corp., Armonk, NY, USA). A p-value of less than 0.05 was considered to indicate statistical significance.
METHODS
- The patient characteristics are summarized in Table 1. The male-to-female ratio was 49:18 and the mean age was 49.5 years (range 8–78 years). Twenty-two of the 67 patients (32.8%) had an Evans index greater than 0.3 on preoperative CT scans, and 38 (56.7%) showed an increasing ventricle size about 1 month after CP. During careful follow-up, only six of these patients (15.7%, 8.9% of enrolled patients) showed worsening neurologic symptoms that were improved by the tap test. These patients underwent VPS (Fig. 2). No complications were reported.
- The mean Evans index of these six patients was 0.361 before they underwent CP (Table 2). The patients’ ventriculomegaly improved somewhat after CP and before VPS placement, as the mean Evans index value decreased to 0.358. The size of the ventricle decreased in two patients, stayed the same in one patient, and increased in three patients after CP.
- No statistically significant differences were found in the degree of ventriculomegaly according to whether patients underwent VPS placement. The largest width of the frontal horn and Evans index were compared, both before and after CP. We were unable to find statistically significant differences in the prevalence of ventriculomegaly (Evans index greater than 0.3) before CP between these two groups (Table 3).
RESULTS
- The mechanism of hydrocephalus after DC was briefly described in the Introduction section, but in more detail, hydrocephalus occurring after DC may be presumed to be due to several factors. These factors have been reported to be 1) neuronal cell loss and severe atrophy of the brain parenchyma due to local destructive or ischemic lesions, 2) adhesive arachnoiditis of the basal cisterns, 3) blood blockage and dysfunction of the arachnoid granules, and 4) internal displacement of the scalp and reduction in CSF flow in the convexity due to the gradient between the atmospheric pressure and the intracranial pressure [10,17,18]. It is also assumed that the size of the ventricle will gradually increase to a certain critical point due to factors 1), 2), and 3), which persist after CP. In this study, 38 out of 67 patients (56.7%) showed ventricular enlargement after CP. However, only six of them developed progressive, clinically evident hydrocephalus. This suggests that CP itself could act as a defense against atmospheric pressure and promote the recovery of CSF flow, thereby exerting a therapeutic effect on hydrocephalus [13,19]. Therefore, radiographic evidence of ventriculomegaly itself cannot be an absolute indication for VPS. Instead, each individual patient’s clinical and neurologic progression must be taken into consideration. As a consequence, we did not proceed with VPS in all patients, even in those with ventriculomegaly. The authors also strongly recommend making a clinical diagnosis through the tap test. A positive response to the withdrawal of CSF has been reported to have a positive predictive value in the range of 73–100% [20–22]. In addition, VPS has well-known complications, such as wound healing problems, bleeding, malfunction, and infections [23,24]. It was also reported that when CP and VPS placement were performed simultaneously, the complication rate was higher than that of staged surgery [11,25].
- In this study, authors were unable to find a statistically significant difference in prevalence of ventriculomegaly before CP or in the degree of ventriculomegaly according to whether patients eventually underwent VPS placement. Furthermore, two patients who did not have ventriculomegaly before CP had a gradual increase in ventricle size on follow-up CT and eventually required a VPS. Therefore, careful neurologic examinations throughout a sufficient follow-up period are advised for every patient who undergoes CP.
- The current study has several limitations. First, this study examined a small number of patients in a retrospective manner. Second, the follow-up period was relatively short. Post-traumatic hydrocephalus is a gradually progressive condition, and therefore much longer-term follow-up is necessary to investigate the progress of hydrocephalus after CP. Further randomized prospective studies with a larger sample size are required to assess the possible indications for VPS placement after DC.
- Nonetheless, our findings suggest that VPS placement is not always required for radiologically diagnosed ventriculomegaly in head trauma patients after DC. A careful clinical and neurologic evaluation should be conducted before placing a VPS.
DISCUSSION
- Our results suggest that a VPS is not necessarily required for ventriculomegaly after DC in TBI patients. Performing both CP and VPS placement can also lead to various complications. Therefore, CP should be performed first and a VPS can be considered after careful observation in selected patients with progressive radiographic findings, worsening neurologic status, and a positive tap test.
CONCLUSION
Fig. 1(A) Computed tomography (CT) images showing progressive ventriculomegaly. The maximal biparietal diameter was checked on the initial CT images. (B, C) The largest width of the frontal horns was measured on similar slices before and after cranioplasty.
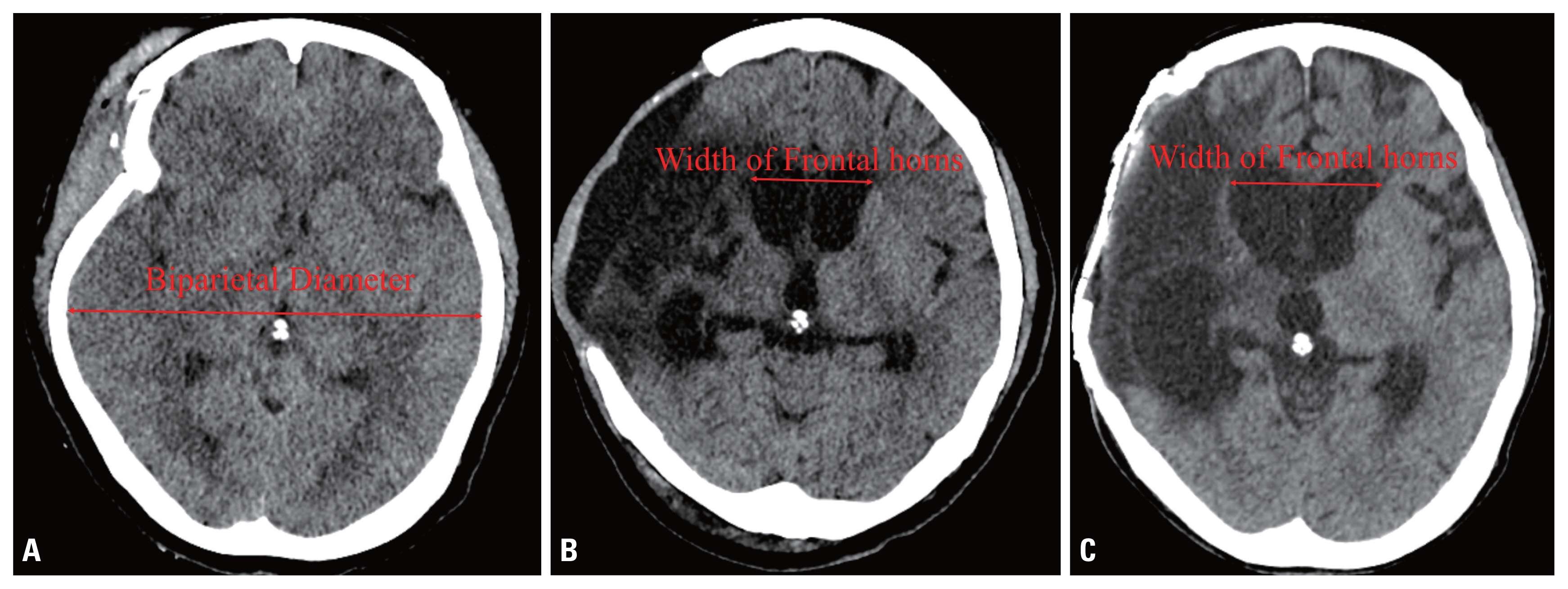

Table 1Characteristics of patients in the present study
Table 2Characteristics and progression of six patients who underwent VPS placement
| Case No. | Age/sex | Evans index | |
|---|---|---|---|
| Before CP | After CP | ||
| 1 | 61/M | 0.160 | 0.222 |
| 2 | 21/M | 0.299 | 0.321 |
| 3 | 65/F | 0.380 | 0.324 |
| 4 | 45/M | 0.382 | 0.382 |
| 5 | 53/F | 0.455 | 0.478 |
| 6 | 24/M | 0.493 | 0.421 |
| Mean | 44.8 | 0.361 | 0.385 |
Table 3Radiographic characteristics of the patients who underwent VPS placement
- 1. Jeong TS, Kim WK, Jang MJ. Cranioplasty results after the use of a polyester urethane dural substitute (Neuro-Patch®) as an adhesion prevention material in traumatic decompressive craniectomy. J Trauma Inj 2019;32:195–201. ArticlePDF
- 2. Kim J, Kim JH, Kim JH, Kwon TH, Roh H. Outcomes of cranioplasty using autologous bone or 3D-customized titanium mesh following decompressive craniectomy for traumatic brain injury: differences in complications. J Trauma Inj 2019;32:202–9. ArticlePDF
- 3. Kim JH, Kim JH, Kwon TH, Chong K, Hwang SY, Yoon WK. Aseptic bone flap resorption after cranioplasty with autologous bone: incidence, risk factors, and clinical implications. World Neurosurg 2018;115:e111–8. ArticlePubMed
- 4. Schuss P, Vatter H, Oszvald A, Marquardt G, Imöhl L, Seifert V, et al. Bone flap resorption: risk factors for the development of a long-term complication following cranioplasty after decompressive craniectomy. J Neurotrauma 2013;30:91–5. ArticlePubMed
- 5. Alvis-Miranda H, Castellar-Leones SM, Moscote-Salazar LR. Decompressive craniectomy and traumatic brain injury: a review. Bull Emerg Trauma 2013;1:60–8. PubMedPMC
- 6. Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, D’Urso P, et al. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med 2011;364:1493–502. ArticlePubMed
- 7. Annan M, De Toffol B, Hommet C, Mondon K. Sinking skin flap syndrome (or Syndrome of the trephined): a review. Br J Neurosurg 2015;29:314–8. ArticlePubMed
- 8. Yang XF, Wang H, Wen L, Huang X, Li G, Gong JB. The safety of simultaneous cranioplasty and shunt implantation. Brain Inj 2017;31:1651–5. ArticlePubMed
- 9. Pachatouridis D, Alexiou GA, Michos E, Voulgaris S. Timing of cranioplasty and shunt placement. Brain Inj 2018;32:529. ArticlePubMed
- 10. Pachatouridis D, Alexiou GA, Zigouris A, Michos E, Drosos D, Fotakopoulos G, et al. Management of hydrocephalus after decompressive craniectomy. Turk Neurosurg 2014;24:855–8. PubMed
- 11. Heo J, Park SQ, Cho SJ, Chang JC, Park HK. Evaluation of simultaneous cranioplasty and ventriculoperitoneal shunt procedures. J Neurosurg 2014;121:313–8. ArticlePubMed
- 12. Dujovny M, Fernandez P, Alperin N, Betz W, Misra M, Mafee M. Post-cranioplasty cerebrospinal fluid hydrodynamic changes: magnetic resonance imaging quantitative analysis. Neurol Res 1997;19:311–6. ArticlePubMed
- 13. Fodstad H, Love JA, Ekstedt J, Fridén H, Liliequist B. Effect of cranioplasty on cerebrospinal fluid hydrodynamics in patients with the syndrome of the trephined. Acta Neurochir (Wien) 1984;70:21–30. ArticlePubMedPDF
- 14. Fodstad H, Ekstedt J, Fridén H. CSF hydrodynamic studies before and after cranioplasty. Acta Neurochir Suppl (Wien) 1979;28:514–8. PubMed
- 15. Gooch MR, Gin GE, Kenning TJ, German JW. Complications of cranioplasty following decompressive craniectomy: analysis of 62 cases. Neurosurg Focus 2009;26:E9. ArticlePMC
- 16. Schuss P, Borger V, Güresir Á, Vatter H, Güresir E. Cranioplasty and ventriculoperitoneal shunt placement after decompressive craniectomy: staged surgery is associated with fewer postoperative complications. World Neurosurg 2015;84:1051–4. ArticlePubMed
- 17. Pollack IF, Albright AL, Adelson PD. A randomized, controlled study of a programmable shunt valve versus a conventional valve for patients with hydrocephalus. Hakim-Medos Investigator Group. Neurosurgery 1999;45:1399–408; discussion 1408–11. ArticlePubMed
- 18. Portnoy HD, Chopp M, Branch C, Shannon MB. Cerebrospinal fluid pulse waveform as an indicator of cerebral autoregulation. J Neurosurg 1982;56:666–78. ArticlePubMed
- 19. Winkler PA, Stummer W, Linke R, Krishnan KG, Tatsch K. Influence of cranioplasty on postural blood flow regulation, cerebrovascular reserve capacity, and cerebral glucose metabolism. J Neurosurg 2000;93:53–61. Article
- 20. Haan J, Thomeer RT. Predictive value of temporary external lumbar drainage in normal pressure hydrocephalus. Neurosurgery 1988;22:388–91. ArticlePubMedPDF
- 21. Malm J, Kristensen B, Karlsson T, Fagerlund M, Elfverson J, Ekstedt J. The predictive value of cerebrospinal fluid dynamic tests in patients with th idiopathic adult hydrocephalus syndrome. Arch Neurol 1995;52:783–9. ArticlePubMed
- 22. Walchenbach R, Geiger E, Thomeer RT, Vanneste JA. The value of temporary external lumbar CSF drainage in predicting the outcome of shunting on normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry 2002;72:503–6. PubMedPMC
- 23. Fernández-Méndez R, Richards HK, Seeley HM, Pickard JD, Joannides AJ, UKSR collaborators. Current epidemiology of cerebrospinal fluid shunt surgery in the UK and Ireland (2004–2013). J Neurol Neurosurg Psychiatry 2019;90:747–54. ArticlePubMed
- 24. Stein SC, Guo W. Have we made progress in preventing shunt failure? A critical analysis. J Neurosurg Pediatr 2008;1:40–7. ArticlePubMed
- 25. Schuss P, Vatter H, Marquardt G, Imöhl L, Ulrich CT, Seifert V, et al. Cranioplasty after decompressive craniectomy: the effect of timing on postoperative complications. J Neurotrauma 2012;29:1090–5. ArticlePubMed
REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

- Post-traumatic hydrocephalus may be associated with autologous cranioplasty failure, independent of ventriculoperitoneal shunt placement: a retrospective analysis
Carole S. L. Spake, Dardan Beqiri, Vinay Rao, Joseph W. Crozier, Konstantina A. Svokos, Albert S. Woo
British Journal of Neurosurgery.2022; 36(6): 699. CrossRef
 KST
KST

 PubReader
PubReader ePub Link
ePub Link Cite
Cite