Articles
- Page Path
- HOME > J Trauma Inj > Volume 34(4); 2021 > Article
-
Case Report
Atypical Hemolytic Uremic Syndrome after Traumatic Rectal Injury: A Case Report -
Ji-Hyoun Kang, M.D., Ph.D.1, Donghyun Lee, B.D.2, Yunchul Park, M.D.2

-
Journal of Trauma and Injury 2021;34(4):299-304.
DOI: https://doi.org/10.20408/jti.2020.0068
Published online: September 1, 2021
- 2,620 Views
- 62 Download
1Division of Rheumatology, Department of Internal Medicine, Chonnam National University Hospital, Chonnam National University Medical School, Gwangju, Korea
2Division of Trauma Surgery, Department of Surgery, Chonnam National University Hospital, Chonnam National University Medical School, Gwangju, Korea
- Correspondence to Yunchul Park, M.D. Division of Trauma Surgery, Department of Surgery, Chonnam National University Hospital, Chonnam National University Medical School, 42 Jebong-ro, Dong-gu, Gwangju 61469, Korea Tel: +82-62-220-5444 Fax: +82-62-220-5444 E-mail: kontikithor@gmail.com
Copyright © 2021 The Korean Society of Traumatology
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- Atypical hemolytic uremic syndrome (aHUS) is a rare, progressive, life-threatening condition of thrombotic microangiopathy characterized by thrombocytopenia, microangiopathic hemolytic anemia, and renal impairment. The mechanisms underlying aHUS remain unclear. Herein, we present the first case in the literature of aHUS after a traumatic injury. A 55-year-old male visited the emergency department after a traumatic injury caused by a tree limb. Abdominal computed tomography revealed a rectal wall defect with significant air density in the perirectal space and preperitoneum, implying rectal perforation. Due to the absence of intraperitoneal intestinal perforation, we performed diverting sigmoid loop colostomy. An additional intermittent simple repair was performed due to perianal and anal injuries. One day postoperatively, his urine output abruptly decreased and serum creatinine level increased. His platelet level decreased, and a spiking fever occurred after 2 days. The patient was diagnosed with acute renal failure secondary to aHUS and was treated with fresh frozen plasma replacement. Continuous renal replacement therapy (CRRT) was also started for oliguria and uremic symptoms. The patient received CRRT for 3 days and intermittent hemodialysis thereafter. After hemodialysis and subsequent supportive treatment, his urine output and renal function improved. The hemolytic anemia and thrombocytopenia also gradually improved. Dialysis was terminated on day 22 of admission and the patient was discharged after recovery. This case suggests that that a traumatic event can trigger aHUS, which should be considered in patients who have thrombocytopenia and acute renal failure with microangiopathic hemolytic anemia. Early diagnosis and appropriate management are critical for favorable outcomes.
- Atypical hemolytic uremic syndrome (aHUS) is a rare, progressive, life-threatening condition of thrombotic microangiopathy (TMA). This disease is characterized by an abrupt or gradual onset of thrombocytopenia, microangiopathic hemolytic anemia (MAHA), and renal impairment [1-3], and can occur at any age. The mechanisms of aHUS remain unknown. However, this disease has a poor prognosis and a higher morbidity and mortality than typical HUS. Thus, untreated aHUS remains a lifelong risk factor for impaired renal function, end-stage renal disease, extrarenal manifestations, and death [4-7].
- The etiology of TMA is important to explain the mechanism of aHUS. TMA is mainly caused by dysregulation of the alternative pathway of the complement system. Aggravating conditions such as pregnancy complications, malignant hypertension, autoimmune diseases, and organ or hematopoietic stem-cell transplantations are associated with the onset of TMA. To the best of our knowledge, no report has yet described the onset of aHUS after traumatic injury. Here, we present a case in which the patient developed acute renal failure, thrombocytopenia, and TMA following a traumatic injury. Therefore, the findings suggest that aHUS may occur after a traumatic injury, and this information may contribute to the understanding, diagnosis, treatment, and prognosis of this rare disease. The patient provided and signed informed consent for the publication of this case.
INTRODUCTION
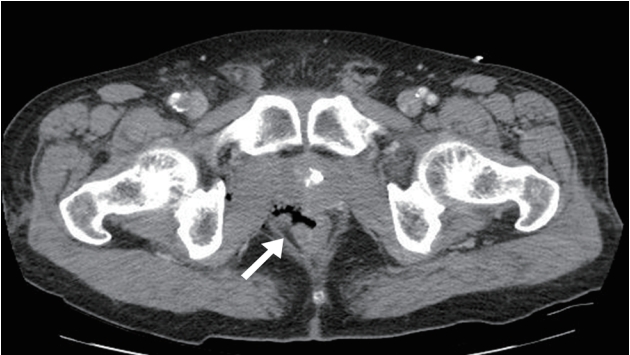
- A 55-year-old male visited the emergency department after a traumatic injury caused by a tree limb. On arrival, he was alert, with a blood pressure of 100/60 mmHg, pulse rate of 72/minute, respiratory rate of 18 breaths/minute, and body temperature of 37.0°C. He had a medical history of percutaneous coronary intervention due to ST-elevation myocardial infarction 2 years ago. A physical examination revealed a long laceration from the perianal area into the rectal wall. Abdominal computed tomography revealed a rectal wall defect with significant air density in the perirectal space and preperitoneum, implying rectal perforation (Fig. 1). An emergency operation was performed. Diagnostic laparoscopic findings revealed a hemorrhagic contusion in the preperitoneal and retroperitoneal space and a small amount of blood in the intraperitoneal space of the pelvic cavity. As there was an absence of intraperitoneal intestinal perforation, we performed diverting sigmoid loop colostomy. An additional intermittent simple repair was performed due to perianal and anal injuries. One day after the operation, the patient’s urine output abruptly decreased and serum creatinine level increased. In addition, his platelet level decreased and a spiking fever occurred after 2 days.
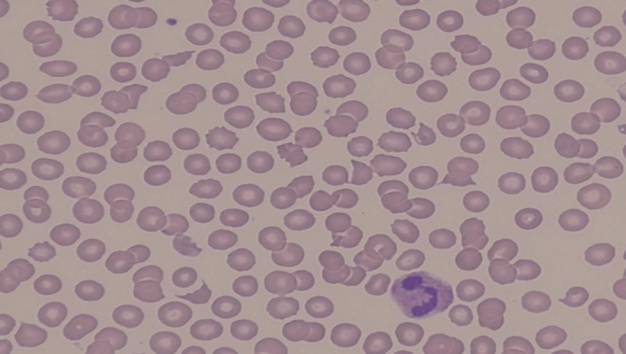
- The laboratory findings upon admission to the emergency room were as follows (Table 1): serum creatinine 1.1 mg/dL; blood urea nitrogen 22.1 mg/dL; total bilirubin 0.4 g/dL; lactate dehydrogenase 440 U/L; and a complete blood count (CBC) showing levels of hemoglobin at 13.4 g/dL, white blood cells (WBCs) at 14.8×103/µL, and platelets at 203×103/µL. Urinalysis revealed clear yellow urine with negative findings for protein, red blood cells, and WBC per high-power field. However, the laboratory findings 2 days after the operation suddenly changed as follows (Table 1): serum creatinine 2.6 mg/dL; blood urea nitrogen 69.8 mg/dL (Chronic Kidney Disease Epidemiology Collaboration estimated glomerular filtration rate [CKD-EPI eGFR] 25.5 mL/minute/1.73 m2); total bilirubin 2.6 g/dL; direct bilirubin 0.1 g/dL; lactate dehydrogenase 1,296 U/L; creatinine kinase 549 U/L; myoglobin 224.0 ng/mL; troponin I 0.2 ng/mL; and haptoglobin <25.8 mg/dL. Arterial blood gas analysis revealed a pH of 7.4, pCO2 of 32.2 mmHg, pO2 of 74.5 mmHg, oxygen saturation of 94.6%, and bicarbonate level of 17.9 mmol/L. CBC showed levels of hemoglobin at 10.9 g/dL, WBCs at 6.1×103/µL, platelets at 45×103/µL, and a high reticulocyte count at 1.4%. The WBC differential count showed neutrophils at 93.7%, eosinophils at 1.9%, lymphocytes at 4.0%, and monocytes at 0.4%. The prothrombin time and partial thromboplastin time were 1.0 (international normalized ratio) and 23.9 seconds, respectively. The levels of fibrin degradation products, fibrinogen, and D-dimer were 17.5 µg/mL, 469.2 mg/dL, and 3.15 mg/L fibrinogen equivalent unit (FEU), respectively. A peripheral blood smear revealed 3 schistocytes per high-power field (Fig. 2). A serum complement assay showed a C3 level at 78.2 mg/dL and a C4 level at 21.6 mg/dL. Urinalysis revealed dark brown urine with 3+ protein, >100 red blood cells, and 10–19 WBCs per high-power field, along with positive results for hemoglobin. Renal ultrasonography showed normal echogenicity in both kidneys, with right and left renal sizes of 9.6×4.7 cm and 9.1×4.9 cm, respectively.
- According to the diagnostic criteria of aHUS [8], the patient was diagnosed with acute renal failure secondary to aHUS because his presentation satisfied the classic triad of MAHA, thrombocytopenia, and renal failure, with the exclusion of other infections, drugs, or other autoimmune diseases. The patient was treated with fresh frozen plasma replacement. Continuous renal replacement therapy (CRRT) was also started for oliguria and uremic symptoms such as nausea, vomiting, and dyspnea. The patient received CRRT for 3 days and intermittent hemodialysis thereafter. He received four weekly sessions of hemodialysis for 2 weeks. Blood culture and stool culture for Escherichia coli O157 were negative. After hemodialysis and subsequent supportive treatment, his urine output and renal function steadily improved. Similarly, his hemolytic anemia and thrombocytopenia also gradually improved. Dialysis was terminated on day 22 of admission. Finally, the patient was discharged with a creatinine level of 0.7 mg/dL and a platelet count of 286×103/µL.
- At a 1-month follow-up after discharge, the patient was in good health with normal CBC and renal function and had undergone colostomy repair. His improvement has since been maintained. His renal function has also remained normal.
CASE REPORT
- aHUS is an extremely rare disease, with an incidence estimated to be about 0.2–0.4 cases per million. Its pathophysiological mechanism remains unclear. However, it is known that aHUS develops through induced activation of the uncontrolled alternative complement pathway. In addition, the genetic basis of aHUS has been elucidated. Approximately 40–60% of patients with aHUS were found to have genetic or acquired dysregulation of the alternative complement pathway [9,10]. Although the distinction between cause and trigger is not fully evident, coexisting diseases are acquired predisposing factors for aHUS [11]; these can include autoimmune diseases such as systemic lupus erythematosus, systemic sclerosis, transplantation, cancer, infection, pregnancy, or certain cytotoxic drugs [12]. In the steady state, regulation of the complement pathway has significant functional redundancy, but is well tolerated. However, an intercurrent infection involving some other trigger factors activates the complement pathway through an alternative pathway; in particular, the deficiency of a complement regulator can be crucial [13]. Here, to the best of our knowledge, we report the first case of aHUS occurring in a trauma patient, thereby helping to clarify the pathogenesis of aHUS.
- Some hypotheses support this result. First, trauma-induced complement-mediated lysis might be a predisposing factor of aHUS. Complement-mediated lysis, either as a primary or secondary cause, is one of the main mechanisms underlying hemolysis with schistocytes and fragmented red cell in the peripheral blood in aHUS [3,14]. In a previous study, widespread complement activation after trauma culminated in the dysregulation of complement and excessive complement activation products [15]. Thus, it is possible to hypothesize that activation of the complement system after a traumatic injury may have led to the occurrence of aHUS in this case. Further studies will be needed to identify the causal relationship between complement-mediated lysis and trauma. Second, aHUS may be triggered by a hidden infection, as shown by several reports of aHUS being triggered by infections, such as cytomegalovirus, influenza virus, parvovirus B19, upper respiratory tract infections, and gastrointestinal infections [9,16,17]. However, in this case, we did not detect any pathogens in the culture exam. Because empirical antibiotics had already been prescribed due to a mid-rectum laceration with perforation, it is possible that a coexisting gastrointestinal infection was not revealed by the examination.
- The clinical manifestations of aHUS include MAHA in the presence of fragmented erythrocytes in a peripheral blood smear, thrombocytopenia, and acute kidney injury. In clinical practice, patients with simultaneous acute renal failure and thrombocytopenia are often diagnosed with sepsis. In this case, we suspected the patient to have sepsis and prescribed intravenous antibiotics. However, the response to antibiotics was not sufficient, and we observed aggravated laboratory findings and clinical manifestations. Despite maintaining the same antibiotics, the patient improved through the management of aHUS. Thus, it is important to pay attention to whether patients have hemolytic fragmented erythrocytes in the peripheral blood smear.
- aHUS is a highly lethal disease, with a mortality rate of approximately 15% in the acute phase and up to 50% in patients with progressive end-stage renal disease. This is especially true for untreated aHUS, which has a greater risk of increasing morbidity and mortality. The standard treatment includes plasma exchange, hemodialysis, and eculizumab. A recent systematic review of aHUS found no significant difference in outcomes between the eculizumab group and the non-eculizumab group [18]. Even though lifelong eculizumab treatment is recommended in some patients with aHUS, we did not prescribe eculizumab and the patient’s condition improved through plasma replacement and hemodialysis with supportive care.
- There are several limitations to this report. We could not examine possible genetic mutations, such as those affecting complement factor, membrane cofactor protein, thrombomodulin, or ADAMTS 13 activity in this patient. Since the patient did not have neurologic symptoms and the clinical manifestations of aHUS progressed rapidly, the patient’s clinical manifestations were managed first. A traumatic event may trigger aHUS. Therefore, aHUS should be considered in patients who have thrombocytopenia and acute renal failure with MAHA after trauma. This case suggests that early diagnosis and appropriate management can be critical to achieve favorable outcomes in posttraumatic patients with aHUS.
DISCUSSION
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
INFORMED CONSENT
This type of study does not require informed consent.
ARTICLE INFORMATION

Hemoglobin (normal range 12–18 g/dL), platelet count (normal range [130–450]×103/μL), white blood cell count (normal range [4.8–10.8]×103/μL), blood urea nitrogen (normal range 8–23 mg/dL), creatinine (normal range 0.5–1.3 mg/dL), total bilirubin (normal range 0.2–1.3 mg/dL), lactate dehydrogenase (normal range 218–472 U/L), haptoglobin (normal range 30–200 mg/dL), C3 (normal range 90–180 mg/dL), and C4 (normal range 10–30 mg/dL).
aHUS: atypical hemolytic uremic syndrome, WBC: white blood cells, RBC: red blood cells.
- 1. Schmidtko J, Peine S, El-Housseini Y, Pascual M, Meier P. Treatment of atypical hemolytic uremic syndrome and thrombotic microangiopathies: a focus on eculizumab. Am J Kidney Dis 2013;61:289–99. ArticlePubMed
- 2. Caprioli J, Noris M, Brioschi S, Pianetti G, Castelletti F, Bettinaglio P, et al. Genetics of HUS: the impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome. Blood 2006;108:1267–79. ArticlePubMedPMC
- 3. Laurence J. Atypical hemolytic uremic syndrome (aHUS): making the diagnosis. Clin Adv Hematol Oncol 2012;10(10 Suppl 17):1–12.
- 4. Constantinescu AR, Bitzan M, Weiss LS, Christen E, Kaplan BS, Cnaan A, et al. Non-enteropathic hemolytic uremic syndrome: causes and short-term course. Am J Kidney Dis 2004;43:976–82. ArticlePubMed
- 5. Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med 2009;361:1676–87. ArticlePubMed
- 6. Besbas N, Karpman D, Landau D, Loirat C, Proesmans W, Remuzzi G, et al. A classification of hemolytic uremic syndrome and thrombotic thrombocytopenic purpura and related disorders. Kidney Int 2006;70:423–31. ArticlePubMed
- 7. Scheiring J, Andreoli SP, Zimmerhackl LB. Treatment and outcome of Shiga-toxin-associated hemolytic uremic syndrome (HUS). Pediatr Nephrol 2008;23:1749–60. ArticlePubMedPMC
- 8. Laurence J, Haller H, Mannucci PM, Nangaku M, Praga M, Rodriguez de Cordoba S. Atypical hemolytic uremic syndrome (aHUS): essential aspects of an accurate diagnosis. Clin Adv Hematol Oncol 2016;14(Suppl 11):2–15. PubMed
- 9. Noris M, Caprioli J, Bresin E, Mossali C, Pianetti G, Gamba S, et al. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol 2010;5:1844–59. ArticlePubMedPMC
- 10. Fremeaux-Bacchi V, Fakhouri F, Garnier A, Bienaimé F, Dragon-Durey MA, Ngo S, et al. Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults. Clin J Am Soc Nephrol 2013;8:554–62. ArticlePubMedPMC
- 11. Jokiranta TS. HUS and atypical HUS. Blood 2017;129:2847–56. ArticlePubMedPMC
- 12. Loirat C, Fakhouri F, Ariceta G, Besbas N, Bitzan M, Bjerre A, et al. An international consensus approach to the management of atypical hemolytic uremic syndrome in children. Pediatr Nephrol 2016;31:15–39. ArticlePubMed
- 13. Raina R, Krishnappa V, Blaha T, Kann T, Hein W, Burke L, et al. Atypical hemolytic-uremic syndrome: an update on pathophysiology, diagnosis, and treatment. Ther Apher Dial 2019;23:4–21. ArticlePubMed
- 14. Ruggenenti P, Noris M, Remuzzi G. Thrombotic microangiopathy, hemolytic uremic syndrome, and thrombotic thrombocytopenic purpura. Kidney Int 2001;60:831–46. ArticlePubMed
- 15. Huber-Lang M, Gebhard F, Schmidt CQ, Palmer A, Denk S, Wiegner R. Complement therapeutic strategies in trauma, hemorrhagic shock and systemic inflammation - closing Pandora’s box? Semin Immunol 2016;28:278–84. ArticlePubMed
- 16. Weitz M, Amon O, Bassler D, Koenigsrainer A, Nadalin S. Prophylactic eculizumab prior to kidney transplantation for atypical hemolytic uremic syndrome. Pediatr Nephrol 2011;26:1325–9. ArticlePubMed
- 17. Nester C, Stewart Z, Myers D, Jetton J, Nair R, Reed A, et al. Pre-emptive eculizumab and plasmapheresis for renal transplant in atypical hemolytic uremic syndrome. Clin J Am Soc Nephrol 2011;6:1488–94. ArticlePubMedPMC
- 18. Krishnappa V, Gupta M, Elrifai M, Moftakhar B, Ensley MJ, Vachharajani TJ, et al. Atypical hemolytic uremic syndrome: a meta-analysis of case reports confirms the prevalence of genetic mutations and the shift of treatment regimens. Ther Apher Dial 2018;22:178–88. ArticlePubMed
REFERENCES
Figure & Data
References
Citations

 KST
KST

 PubReader
PubReader ePub Link
ePub Link Cite
Cite